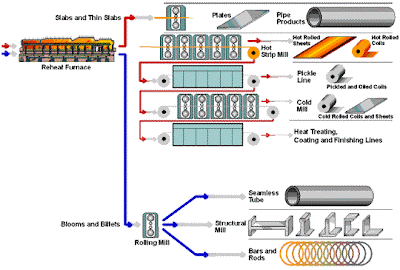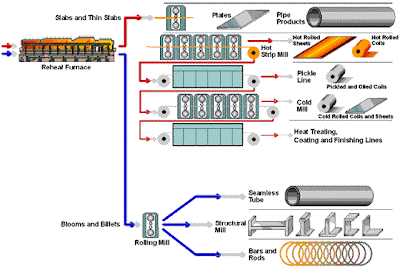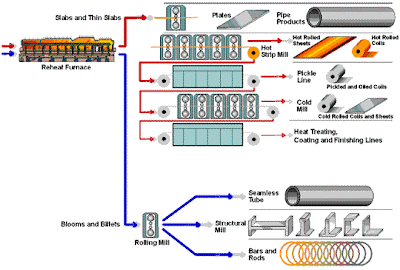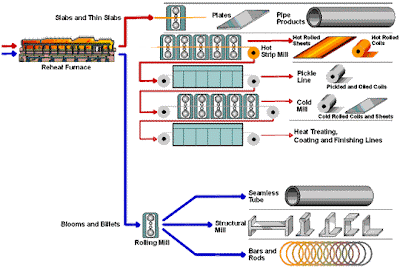
Beneficiation is a variety of processes whereby extracted ore from mining is reduced to particles that can be separated into mineral and waste, the former suitable for further processing or direct use.
The beneficiation process improves the chemical or physical properties of the ore so that metal may be recovered profitably.
Based on this metaphor, the term has come to be used within an economic development and CSR (corporate social responsibility) context - to describe the proportion of the value derived from asset exploitation which stays 'in country' and benefits local communities.
For example, in the diamond industry, the beneficiation imperative argues that cutting and polishing processes within the diamond value-chain should be conducted in-country to maximize the local economic contribution
Iron Ore Trace Elements: Effects and Remedies (Silicon, Phosphorus, Aluminum and Sulfur)
The inclusion of even small amounts of some elements can have profound effects on the behavioral characteristics of a batch of iron or the operation of a smelter.
These effects can be both good and bad. Some catastrophically bad.
Some chemicals were deliberately added. The addition of a flux made a blast furnace more efficient.
Others were added because they made the iron more fluid, harder, or some other desirable quality.
The choice of ore, fuel, and flux determined how the slag behaved and the operational characteristics of the iron produced.
Ideally iron ore contains only iron and oxygen. In nature this is rarely the case. Typically, iron ore contains a host of elements which are often unwanted in modern steel.
Silicon
The major effect of silicon is to promote the formation of gray iron. Gray iron is less brittle and easier to finish than white iron. It was preferred for casting purposes for this reason. Turner (1900:192-7) reported
that silicon also reduced shrinkage and the formation of blowholes, lowering the number of bad castings.
Silica (SiO2) is almost always present in iron ore. Most of it is slagged off during the smelting process.
But, at temperatures above 1300/C some will be reduced and form an alloy with the iron.
The hotter the furnace, the more silicon will be present in the iron.
It is not uncommon to find up to 1.5% Si in European cast iron from the 16th to 18th centuries.
Phosphorus
Turner felt the ideal iron had 0.2-0.55% phosphorus
Phosphorus is a deleterious contaminant because it makes steel brittle, even at concentrations of as little as 0.5%. cannot be easily removed by fluxing or smelting, and so iron ores must generally be low in phosphorus to begin with.
The iron pillar of India which does not rust is protected by a phosphoric composition.
Phosphoric acid is used at a rust converter because phosphoric iron is less susceptible to oxidation.
Aluminum
Aluminum Is very hard to reduce. As a result aluminum contamination of the iron is not a problem.
However, it does increase the viscosity of the slag (Kato and Minowa 1969:37 and
Rosenqvist 1983:311). This will have a number of adverse effects on furnace operation. The thicker slag
will slow the descent of the charge, prolonging the process.
High aluminum will also make it more difficult to tap off the liquid slag. At the extreme this could lead to a frozen furnace.
There are a number of solutions to a high aluminum slag. the first is avoidance, don't
use ore or a lime source with a high aluminum content. Increasing the ratio of lime flux will decrease the viscosity (Rosenqvist 1983:311).
Sulfur
Sulfur dissolves readily in both liquid and solid iron at the temperatures present in iron smelting. The effects of even small amounts of sulfur are immediate and serious. Today iron with over 0.03% sulfur is avoided Sulfur causes iron to be red or hot short (Gordon1996:7)
Hot short iron is brittle when hot.
This was a serious problem as most iron used during the 17th and 18th century was bar or wrought iron.
Wrought iron is shaped by repeated blows with a hammer while hot. A piece of hot short iron will crack if worked with a hammer. When a piece of hot iron or steel cracks the exposed surface immediately oxidizes. This layer of oxide prevents the mending of the crack by welding.
Large cracks cause the iron or steel to break up. Smaller cracks can cause the object to fail during use.
The degree of hot shortness is in direct proportion to the amount of sulfur present.
Today iron with over 0.03% sulfur is avoided. In modern operations, sulfur is unwanted because it produces undesirable sulfur dioxide gases in the flue emissions from a smelter and interferes with the smelting process. Sulfur (S) is a frequent contaminant in coal and coke.
Iron sulfide (pyrite, FeS2), is a common iron ore. It is also present in small quantities in many ores.
It was the presence of sulfur that prevented the use of coal in blast furnaces until
1709.
They were one of the first worked out by iron makers.
To convert an oxide of iron to metallic iron it must be smelted or sent through a direct reduction
process Chemical reduction, or smelting, is a form of extractive metallurgy.
The main use of smelting is to produce a metal from its ore.
This includes iron extraction (for the production of steel) from iron ore, and copper extraction and other base metals from their ores.
It makes use of a chemical reducing agent, commonly a fuel that is a source of carbon such as coke, or in earlier times charcoal, to change the oxidation state of the metal ore; however, plants for the electrolytic reduction of aluminum are also generally referred to as smelters.
The carbon or carbon monoxide derived from it removes oxygen from the ore to leave the metal.
The carbon is oxidized, producing carbon dioxide and carbon monoxide.
As most ores are impure, it is often necessary to use flux, such as limestone to remove the accompanying rock gangue as slag (also called scoria or cinder).
Pre Test Review: Iron Ore Beneficiation & Trace Elements
1.
Beneficiation Is a variety of processes whereby extracted ore from mining is reduced to particles that can be separated into mineral and waste,the former suitable for further processing or direct use.
2.
Chemical reduction, or smelting, is a form of extractive metallurgy.
3.
To convert an oxide of iron to metallic iron it must be smelted or sent through a direct reduction process. The main use of smelting is to produce a metal from its ore.This includes iron extraction (for the production of steel) from iron ore.
4.
Most ores are impure, it is often necessary to use flux, such as limestone to remove the accompanying rock gangue as slag (also called scoria or cinder)
5.
The inclusion of even small amounts of some elements can have profound effects on the behavioral characteristics of a batch of iron or the operation of a smelter.
6.
Some chemicals are deliberately added; Others are added because they make the iron more fluid, harder, or some other desirable quality.
7.
Ideally iron ore contains only iron and oxygen but in nature this is rarely the case. Typically, iron ore contains a host of elements which are often unwanted in modern steel.
8.
The major effect of silicon is to promote the formation of gray iron.which is preferred for casting purposes.
9.
Silicon also reduced shrinkage and the formation of blowholes, lowering the number of bad castings.
10.
Gray Iron is less brittle and easier to finish than white iron.
11.
The hotter the furnace, the more silicon will be present in the iron. It is not uncommon to find up to 1.5% Si in European cast iron from the 16th to 18th centuries.
12.
Silica (SiO2) is almost always present in iron ore. Most of it is slagged off during the smelting process. But, at temperatures above 1300/C some will be reduced and form an alloy with the iron.
13.
Phosphorus is a deleterious contaminant because it makes steel brittle, even at concentrations of as little as 0.5%. cannot be easily removed by fluxing or smelting.
14.
the ideal iron should contain no more than 0.2-0.55% phosphorus
15.
Phosphoric acid is used at a rust converter because phosphoric iron is less susceptible to oxidation. The iron pillar of India which does not rust is protected by a phosphoric composition.
16.
Aluminum is very hard to reduce. As a result aluminum contamination of the iron is not a problem.
17.
Aluminum increases the viscosity of the slag resulting in a number of adverse effects on furnace operation.
18.
High aluminium makes it more difficult to tap off the liquid slag which can lead to a frozen furnace.
19.
There are a number of solutions to a high aluminum slag. the first is avoidance, don't use ore or a lime source with a high aluminum content. Increasing the ratio of lime flux will decrease the viscosity.
20.
The effects of even small amounts of sulfur are immediate and serious.
21.
Today iron with over 0.03% sulfur is avoided
22.
Sulfur causes iron to be red or hot short. The degree of hot shortness is in direct proportion to the amount of sulfur present.
23.
A piece of hot short iron will crack if worked with a hammer. When a piece of hot iron or steel cracks the exposed surface immediately oxidizes. This layer of oxide prevents the mending of the crack by welding.
24.
Large cracks cause the iron or steel to break up. Smaller cracks can cause the object to fail during use.
25.
Iron sulfide (pyrite, FeS2), is a common iron ore. It is also present in small quantities in many ores.
Sample Offer for Sale of Iron Ore
Iron Ore 63% Fe of Loei province, Thailand North Eastern origin for serious inquiries only. Detailed Product Description Iron ores are minerals from which metallic iron is extracted.
Most important use of iron is in the blast furnace for the production of pig iron. It is used in the furnace in the form of sinters and pellets as also lumpy ore. It is also consumed in the open health furnaces.
IRON ORE HANDLING
The design of the processing circuit is designed to feature a jaw crusher equipped with a rock-breaker followed by a two stage crushing and screening operation with horizontal washing screen to produce both lump and fines 0-50 mm size or as client's request. Capacity at 1,000 Mt per day and plan for 4,000 mt
Sufficient stockpiles of ore will be maintained at the processing facilities to allow for uninterrupted trucking/barging to Ayutthaya/Sriracha port.
Specifications;
Iron as Fe: 63 % min : Volumatic test method
Sulfur as S: 0.02%-0.08% max :
Phosphorus as P: 0.02%-0.05% max : Based on ASTM E278-01
Silica as SiO2: 2% to 3.5% max : Gravimatric test method
Aluminum as Al2O3: 0.98%-3.5% max : ICP test method
Specific gravity from 4.0 to 4.5.
Moisture content: Humidity at 105 deg.c on received basis 8%, Rainy season Max.10%.
More information: Iron Ores Chemical Analysis Sizes: Fine and Lump Fines are defined as iron ore with the majority of Individual particles measuring less
than 10 millimeters diameter. Conversely, lump is iron ore Majority of individual particles measuring more than 10 Millimeters diameter. Iron ore lumps varies from + 10 mm to + 75 mm.
More information: Iron ores size control
Fe Content: Iron of as high-grade as possible is required because an increased of 1% Fe in the burden increased the productivity by 2% and decreased the coke rate by 3%. In the THB Mining, the range of the Fe content in iron ore lumps is 60.52% to 66.5%.
Silica: 1.5% decreased of silica causes reduction in the slag volume of 65kg per ton of pig iron. Increase of 100-kg slag per tonne of pig iron raised fuel consumption by 40 kg of coke per tonne of pig iron. In the Thailand Plants, the range of silica in iron ore lumps varies from 1.6% (max.) to 3.5% (max.).
Alumina: If the ore is high in alumina, the fluidity of slag is affected. It should not also be too low. In the Indian Plants, alumina content in the ore varies from 3% to 3.5% Generally.
Phosphorus should not exceed 0.05%
DISTANCE ADVANTAGE:
The closest distance to China, Korea and Japan than other iron ore deposits, the transportation of the iron ore to China and Japan requires shorter time than other exporters. (Approximately 2,600 Nautical miles, India approximately 4,000 Nautical miles, Australia approximately 3,600 nautical miles, and Brazil approximately 10,000 nautical miles to China)
LOCATION OF anywhere PROVINCE, IRON ORES DEPOSITS
==============================================================
Chemical Analysis / per our standards
Iron as Fe - Iron Oxide Fe2O3
As a commercial mineral Iron Ore often also contains small amounts of the following:
Silica as Si - Silicon Dioxide SiO2 :
Silica occurs commonly in nature as sandstone, silica sand or quartzite and ores.
Method of analysis: -Spectrophotometer*
Aluminum as Ag - Aluminum Oxide Al2O3 :
Aluminum oxide is an amphoteric oxide of aluminum with the chemical formula
Al2O3. It is also commonly referred to as alumina in the mining, ceramic and materials science communities. Method of analysis: -Titrimetry (fluorine method)
Titanium as Ti - Titanium Dioxide TiO2 :
Titanium dioxide, also known as titanium(IV) oxide or titania, is the naturally
occurring oxide of titanium, chemical formula TiO2. Method of analysis:
-Spectrophotometer*
Magnesium Mg - Magnesium Oxide MgO
Magnesium is a white solid mineral that occurs naturally as periclase and is a source
of magnesium. Method of analysis: -Spectrophotometer*
Manganese Mn - Manganese Oxide MnO Manganese is a chemical element that has the symbol Mn and atomic number 25. It is found as the free element in nature, often in combination with iron, and in many minerals. Method of analysis: Spectrophotometer*
Sulfur S - Sulfur dioxide SO2 (also sulphur dioxide)
Sulphur is the chemical compound with the formula SO2. This important gas is the main product from the combustion of sulfur. Method of analysis: Gravimetry*
Calcium as Ca - Calcium oxide CaO
Calcium commonly known as lime. Method of analysis: Spectrophotometer*
Loss On Ignition - LOI
Whilst it is desirable to have low contaminant levels of the elements mentioned above, it is considered the opposite for an LOI measure. Essentially, the LOI is a measure of the water content of the ore, which evaporates when the ore is fed into a blast furnace. A typical iron ore analysis should include an LOI determination at 1000 deg. C, normally undertaken by Thermo gravimetric Analyzer (TGA).
*Method accredited: international level for accreditation web site see http://www.cengeolab.com/argaCh_e.htm
2009/05/10
OPPORTUNITIES IN THE GLOBAL STEEL SECTOR
In spite of the most serious U.S. and global economic crisis in decades, the steel industry is poised for recovery -- when demand picks up, James L. Wainscott, American Iron and Steel Institute Chairman (AISI) Chairman, and chairman, president and CEO of AK Steel Corporation, said at the Institute’s annual meeting. With the meeting’s theme, Global Challenges – Global Solutions, leaders of the North American steel industry are meeting here to discuss the state of the global steel sector, market outlooks and challenges, opportunities that will shape the industry’s future and market trends that will be important to steel in the next 12 to 24 months.
“It is important to understand that this recession is not something unique to America or to the North American steel sector,” Wainscott noted. “It is a global recession and our recovery is tied, at least in part, to economic recovery across the world.” He continued, “While we are hopeful that the markets we serve are at, or very near, the trough of the recession, for meaningful economic growth, we’re going to need to see several things: 1) Improved credit availability, 2) The return of consumer confidence, and 3) Rational government policy that serves as a catalyst for growth in American manufacturing and American jobs.”
Wainscott said AISI and its member companies believe that as federal agencies gear-up to use the stimulus funds that have been approved, “they need to ensure that federally-funded projects use American-made iron, steel and other manufactured products, if they are readily available and competitive. This in turn will be beneficial not just to the steel industry but also for the economy to turn the corner as American families and communities depend on a strong manufacturing base to improve quality of life and to create high-paying jobs.”
A recent Department of Transportation study reported that every $1 billion invested in federal highways supports nearly 35,000 American jobs. And a recent economists’ report states that a concerted domestic procurement program could increase U.S. manufacturing job creation by 33 percent while ensuring that taxpayer dollars actually go directly to job creation.
Wainscott raised two main policy issues in his speech – climate change and trade – that have a phenomenal impact on the economy and the steel industry.
“Our industry is firmly committed to building a sustainable future for generations of Americans to come. But our ability to stay competitive in the world economy means we need fair and strong trade and climate laws that are rigorously enforced. In a market open to imports, such as ours, any legislation that undermines the competitiveness of U.S. mills will encourage steel production to leave this market in favor of markets with lower environmental standards,” he said, which would hurt both the U.S. economy and the environment.
He noted that any U.S. climate change regulations involving the steel industry must also provide that similar measures be taken by other major steel-producing countries such as China.
“It is a known fact that countries like China will not adopt measures to curb CO2 emissions as aggressively as we will, which will give them and other importers a tremendous competitive advantage. That is, unless U.S. climate policy provides offsets and border adjustment mechanisms to account for the cost burden placed on U.S. companies,” he emphasized.
America’s steel industry has achieved sizable CO2 reductions and the processes are limited by the laws of physics regarding further energy and CO2 reductions. In contrast, a study released by the Alliance for American Manufacturing shows that China is now responsible for at least 50 percentof total global steelmaking CO2 emissions generated, he said.
“It’s clear to me that, if we don’t get our climate policy right, we face the prospect of ceding another huge segment of America’s industrial prowess to the BRIC countries – Brazil, Russia, India and China. And that would mean further loss of our manufacturing jobs,” said Wainscott.
On trade issues, he emphasized the need for “enforcing trade laws and for our government to stand-up for our nation’s assets – our workers and manufacturing sector.”
“The issue of China’s rampant currency manipulation cannot be ignored. The Chinese government does not play by the rules when it illegally subsidizes its industry and controls its currency's exchange rate,” Wainscott added. “These actions enable China to evade market forces and falsely gain a 40 percent competitive advantage over U.S.-manufactured goods, which has greatly contributed to America's $256 billion trade deficit with China,” he said.
In a call to action, Wainscott urged Congress to enact legislation that treats China’s currency manipulation as a subsidy under our trade remedy laws.
Concluding his speech on a positive note, Wainscott said that despite these challenges, he is optimistic for the future of the steel industry. “My reason for optimism lies in the dynamic spirit of a great workforce, innovative products and an amazing material. We’ve all been through adverse times before and have emerged stronger.”
AISI serves as the voice of the North American steel industry in the public policy arena and advances the case for steel in the marketplace as the preferred material of choice. AISI also plays a lead role in the development and application of new steels and steelmaking technology. AISI is comprised of 24 member companies, including integrated and electric furnace steelmakers, and 138 associate and affiliate members who are suppliers to or customers of the steel industry. AISI's member companies represent approximately 75 percent of both U.S. and North American steel capacity. For more news about steel and its applications,
from: steel.org
“It is important to understand that this recession is not something unique to America or to the North American steel sector,” Wainscott noted. “It is a global recession and our recovery is tied, at least in part, to economic recovery across the world.” He continued, “While we are hopeful that the markets we serve are at, or very near, the trough of the recession, for meaningful economic growth, we’re going to need to see several things: 1) Improved credit availability, 2) The return of consumer confidence, and 3) Rational government policy that serves as a catalyst for growth in American manufacturing and American jobs.”
Wainscott said AISI and its member companies believe that as federal agencies gear-up to use the stimulus funds that have been approved, “they need to ensure that federally-funded projects use American-made iron, steel and other manufactured products, if they are readily available and competitive. This in turn will be beneficial not just to the steel industry but also for the economy to turn the corner as American families and communities depend on a strong manufacturing base to improve quality of life and to create high-paying jobs.”
A recent Department of Transportation study reported that every $1 billion invested in federal highways supports nearly 35,000 American jobs. And a recent economists’ report states that a concerted domestic procurement program could increase U.S. manufacturing job creation by 33 percent while ensuring that taxpayer dollars actually go directly to job creation.
Wainscott raised two main policy issues in his speech – climate change and trade – that have a phenomenal impact on the economy and the steel industry.
“Our industry is firmly committed to building a sustainable future for generations of Americans to come. But our ability to stay competitive in the world economy means we need fair and strong trade and climate laws that are rigorously enforced. In a market open to imports, such as ours, any legislation that undermines the competitiveness of U.S. mills will encourage steel production to leave this market in favor of markets with lower environmental standards,” he said, which would hurt both the U.S. economy and the environment.
He noted that any U.S. climate change regulations involving the steel industry must also provide that similar measures be taken by other major steel-producing countries such as China.
“It is a known fact that countries like China will not adopt measures to curb CO2 emissions as aggressively as we will, which will give them and other importers a tremendous competitive advantage. That is, unless U.S. climate policy provides offsets and border adjustment mechanisms to account for the cost burden placed on U.S. companies,” he emphasized.
America’s steel industry has achieved sizable CO2 reductions and the processes are limited by the laws of physics regarding further energy and CO2 reductions. In contrast, a study released by the Alliance for American Manufacturing shows that China is now responsible for at least 50 percentof total global steelmaking CO2 emissions generated, he said.
“It’s clear to me that, if we don’t get our climate policy right, we face the prospect of ceding another huge segment of America’s industrial prowess to the BRIC countries – Brazil, Russia, India and China. And that would mean further loss of our manufacturing jobs,” said Wainscott.
On trade issues, he emphasized the need for “enforcing trade laws and for our government to stand-up for our nation’s assets – our workers and manufacturing sector.”
“The issue of China’s rampant currency manipulation cannot be ignored. The Chinese government does not play by the rules when it illegally subsidizes its industry and controls its currency's exchange rate,” Wainscott added. “These actions enable China to evade market forces and falsely gain a 40 percent competitive advantage over U.S.-manufactured goods, which has greatly contributed to America's $256 billion trade deficit with China,” he said.
In a call to action, Wainscott urged Congress to enact legislation that treats China’s currency manipulation as a subsidy under our trade remedy laws.
Concluding his speech on a positive note, Wainscott said that despite these challenges, he is optimistic for the future of the steel industry. “My reason for optimism lies in the dynamic spirit of a great workforce, innovative products and an amazing material. We’ve all been through adverse times before and have emerged stronger.”
AISI serves as the voice of the North American steel industry in the public policy arena and advances the case for steel in the marketplace as the preferred material of choice. AISI also plays a lead role in the development and application of new steels and steelmaking technology. AISI is comprised of 24 member companies, including integrated and electric furnace steelmakers, and 138 associate and affiliate members who are suppliers to or customers of the steel industry. AISI's member companies represent approximately 75 percent of both U.S. and North American steel capacity. For more news about steel and its applications,
from: steel.org
Iron Ore Processing for the Blast Furnace
The following describes operations at the National Steel Pellet Company, an iron ore mining and processing facility located on the Mesabi Iron Range of Minnesota.
Creating steel from low-grade iron ore requires a long process of mining, crushing, separating, concentrating, mixing, pelletizing, and shipping. The process of mining low-grade iron ore, or taconite, requires massive resources. Heavy industrial mining equipment, expansive mines, and a skilled labor pool are all required. The equipment used includes diamond-bit rotary drills, hydraulic shovels and loaders, water wagons, production trucks and heavy-duty conveyors.
National Steel Pellet Company’s plant is capable of producing 5.35 million tons of pellets each year. It employs approximately 500 workers.
Mining Iron Ore
Mining iron ore begins at ground level. Taconite is identified by diamond drilling core samples on a grid hundreds of feet into the earth. Taconite rock comprises about 28 percent iron; the rest is sand or silica. These samples are analyzed and categorized so that mining engineers can accurately develop a mine plan.
To uncover taconite reserves, the mine area is first "stripped" of the overburden or glacial drift, comprised primarily of rock, clay and gravel. The overburden is loaded by large hydraulic shovels into production trucks, which haul it to contour dumps. These dumps are environmentally designed to match the surrounding area.
Once the taconite rock is exposed, large drilling rigs drill blast holes 16" in diameter by 40' deep, in some cases. Nearly 400 of these holes are drilled in a blast pattern. Before the blast, the holes are filled with a special mixture of blasting agents. Once prepared, the mine site is cleared of workers and equipment, and the blast is detonated. Each of the holes is detonated just a millisecond apart, resulting in a pile of crude taconite that is broken apart to a minus 6' x 6' size.
After blasting, hydraulic face shovels and larger loaders load the taconite into 205-ton or 240-ton production trucks, which haul it to crushers. The taconite is ground to a fine powder and mixed with water. A series of magnets is run over the mixture. The magnets grab the iron particles and the rest is discarded. For every ton of iron retained, two tons of waste, or tailings, are discarded.
Crushing the Ore
The crude taconite is delivered to large gyrator crushers, where chunks as large as five feet are reduced to six inches or less. More than 6,000 tons of taconite can be crushed in one hour.
The crushed material is transferred by belt to an ore storage building, which holds up to 220,000 tons of taconite. An apron feeder sends the ore to the concentrator building for grinding, separating, and concentrating.
Concentrating
The crude taconite is now roughly the size of a football or smaller. A series of conveyor belts continuously feed the ore into ten large 27-foot-diameter, semi-autogenous primary grinding mills. Water is added at this point to transport it (94 percent of the water is recycled, while the rest is lost through evaporation).
Each primary mill contains several 4" steel balls that grind the ore as the mills turn. When the ore is reduced to 3/4" or less, it moves out of the mill in a slurry solution. The mill discharge is screened at 1/4" on trommel screens attached to the mill. Ore smaller than 1/4" is pumped in slurry solution to the wet cobber magnetic separator, which begins the process of separating the iron from the non-iron material. The magnetic iron ore is then laundered in two slurry surge tanks while the non-magnetics (silica/sand) go to the tailings disposal area.
Most of the material continues to be finely ground in one of five secondary ball mills, which are powered by electric motors ranging from 2,500 hp to 4,000 hp and are charged with 1-1/2" chrome grinding balls. Fine grinding is achieved using these smaller mills, bringing the ore to a similar grind as that found in face powder. The screen undersize is then moved to hydroseparators, where silica is floated off the top.
The hydroseparator underflow is pumped to the finisher magnetic separators. Once again, the magnetic separators grab the iron and discard the silica and sand. Thus, the ore is "concentrated" by removing the waste materials. The concentrate from the separators is pumped to fine screening.
The oversize material is returned to the balls mills, while the undersize (with the most impurities removed) becomes the final concentrate. Waste from the circuit goes to the tailings basin and the final concentrate travels to thickeners located in the pellet plant. The underflow from the thickeners is pumped to a storage tank and then to disc filters for dewatering.
The product is called “filter cake”, and is now ready for mixing with the binding agent.
Mixing with the Binding Agents
Once the filter cake is complete, it is deposited into a surge bin. It then travels onto a feeder belt and from there to a conveyor where bentonite, a bonding agent, is added. Bentonite is a clay from Wyoming used to help iron ore concentrate stick together when rolled into pellets. About 16 pounds of Bentonite are added to every ton of iron ore concentrate.
Small amounts of limestone (1%) are also added and mixed with the concentrate at this point. Limestone is added to meet the requirements of steel customers in the blast furnace process.
The iron ore concentrate is now mixed and ready for the pelletizing process.
Pelletizing
A pellet plant contains a series of balling drums where the iron ore concentrate is formed into soft pellets, in much the same manner that one rolls a snowball, to make a pellet about the size of a marble (between 1/4" and 1/2"). Pellets are screened to meet the size specification, with undersized or oversized pellets crushed and returned to the balling drums.
The soft pellets are then delivered to the roller feeder for final removal of the fines, which are also returned to the balling circuits. Now the soft pellets, correctly sized, are delivered to the traveling grate furnace for further drying and preheating. The grate is fired by natural gas.
From this point, the pellets are charged into the large rotary kiln where they are heat-hardened at 2,400 degrees Fahrenheit. The pellets are discharged into the revolving cooler and then moved to the pellet screening plant, onto the pellet loadout system. The whole process consumes energy in the form of electricity and natural gas. Over the past several years, millions of dollars have been spent to improve energy efficiency and to recoup waste heat and re-use it in the process. These efforts have significantly reduced expenditures on energy.
The pelletizing process has now been completed. The pellets are run through a final screening to remove those not meeting size specifications or those that are chipped or broken into fines. Pellets that meet the necessary standards are conveyed to the pellet stockpile, which holds about 30,000 tons.
Pellet Loadout and Shipping
The pellets are now ready for shipping by train to customers or to ore docks. They are sent to blast furnaces and steel mills, where they will be turned into finished steel.
A trainload of iron ore pellets bound for the blast furnace
National Steel Pellet Company’s iron ore pellets have the following characteristics (FOB Mine):
Total Iron: 65.85% Silica (SiO2): 4.5% Lime (CaO): 0.68% Phosphorous: 0.010
Size: %+1/4” 96.5% (after tumble) Compression Strength: 560 pounds
from: http://www.steel.org
Creating steel from low-grade iron ore requires a long process of mining, crushing, separating, concentrating, mixing, pelletizing, and shipping. The process of mining low-grade iron ore, or taconite, requires massive resources. Heavy industrial mining equipment, expansive mines, and a skilled labor pool are all required. The equipment used includes diamond-bit rotary drills, hydraulic shovels and loaders, water wagons, production trucks and heavy-duty conveyors.
National Steel Pellet Company’s plant is capable of producing 5.35 million tons of pellets each year. It employs approximately 500 workers.
Mining Iron Ore
Mining iron ore begins at ground level. Taconite is identified by diamond drilling core samples on a grid hundreds of feet into the earth. Taconite rock comprises about 28 percent iron; the rest is sand or silica. These samples are analyzed and categorized so that mining engineers can accurately develop a mine plan.
To uncover taconite reserves, the mine area is first "stripped" of the overburden or glacial drift, comprised primarily of rock, clay and gravel. The overburden is loaded by large hydraulic shovels into production trucks, which haul it to contour dumps. These dumps are environmentally designed to match the surrounding area.
Once the taconite rock is exposed, large drilling rigs drill blast holes 16" in diameter by 40' deep, in some cases. Nearly 400 of these holes are drilled in a blast pattern. Before the blast, the holes are filled with a special mixture of blasting agents. Once prepared, the mine site is cleared of workers and equipment, and the blast is detonated. Each of the holes is detonated just a millisecond apart, resulting in a pile of crude taconite that is broken apart to a minus 6' x 6' size.
After blasting, hydraulic face shovels and larger loaders load the taconite into 205-ton or 240-ton production trucks, which haul it to crushers. The taconite is ground to a fine powder and mixed with water. A series of magnets is run over the mixture. The magnets grab the iron particles and the rest is discarded. For every ton of iron retained, two tons of waste, or tailings, are discarded.
Crushing the Ore
The crude taconite is delivered to large gyrator crushers, where chunks as large as five feet are reduced to six inches or less. More than 6,000 tons of taconite can be crushed in one hour.
The crushed material is transferred by belt to an ore storage building, which holds up to 220,000 tons of taconite. An apron feeder sends the ore to the concentrator building for grinding, separating, and concentrating.
Concentrating
The crude taconite is now roughly the size of a football or smaller. A series of conveyor belts continuously feed the ore into ten large 27-foot-diameter, semi-autogenous primary grinding mills. Water is added at this point to transport it (94 percent of the water is recycled, while the rest is lost through evaporation).
Each primary mill contains several 4" steel balls that grind the ore as the mills turn. When the ore is reduced to 3/4" or less, it moves out of the mill in a slurry solution. The mill discharge is screened at 1/4" on trommel screens attached to the mill. Ore smaller than 1/4" is pumped in slurry solution to the wet cobber magnetic separator, which begins the process of separating the iron from the non-iron material. The magnetic iron ore is then laundered in two slurry surge tanks while the non-magnetics (silica/sand) go to the tailings disposal area.
Most of the material continues to be finely ground in one of five secondary ball mills, which are powered by electric motors ranging from 2,500 hp to 4,000 hp and are charged with 1-1/2" chrome grinding balls. Fine grinding is achieved using these smaller mills, bringing the ore to a similar grind as that found in face powder. The screen undersize is then moved to hydroseparators, where silica is floated off the top.
The hydroseparator underflow is pumped to the finisher magnetic separators. Once again, the magnetic separators grab the iron and discard the silica and sand. Thus, the ore is "concentrated" by removing the waste materials. The concentrate from the separators is pumped to fine screening.
The oversize material is returned to the balls mills, while the undersize (with the most impurities removed) becomes the final concentrate. Waste from the circuit goes to the tailings basin and the final concentrate travels to thickeners located in the pellet plant. The underflow from the thickeners is pumped to a storage tank and then to disc filters for dewatering.
The product is called “filter cake”, and is now ready for mixing with the binding agent.
Mixing with the Binding Agents
Once the filter cake is complete, it is deposited into a surge bin. It then travels onto a feeder belt and from there to a conveyor where bentonite, a bonding agent, is added. Bentonite is a clay from Wyoming used to help iron ore concentrate stick together when rolled into pellets. About 16 pounds of Bentonite are added to every ton of iron ore concentrate.
Small amounts of limestone (1%) are also added and mixed with the concentrate at this point. Limestone is added to meet the requirements of steel customers in the blast furnace process.
The iron ore concentrate is now mixed and ready for the pelletizing process.
Pelletizing
A pellet plant contains a series of balling drums where the iron ore concentrate is formed into soft pellets, in much the same manner that one rolls a snowball, to make a pellet about the size of a marble (between 1/4" and 1/2"). Pellets are screened to meet the size specification, with undersized or oversized pellets crushed and returned to the balling drums.
The soft pellets are then delivered to the roller feeder for final removal of the fines, which are also returned to the balling circuits. Now the soft pellets, correctly sized, are delivered to the traveling grate furnace for further drying and preheating. The grate is fired by natural gas.
From this point, the pellets are charged into the large rotary kiln where they are heat-hardened at 2,400 degrees Fahrenheit. The pellets are discharged into the revolving cooler and then moved to the pellet screening plant, onto the pellet loadout system. The whole process consumes energy in the form of electricity and natural gas. Over the past several years, millions of dollars have been spent to improve energy efficiency and to recoup waste heat and re-use it in the process. These efforts have significantly reduced expenditures on energy.
The pelletizing process has now been completed. The pellets are run through a final screening to remove those not meeting size specifications or those that are chipped or broken into fines. Pellets that meet the necessary standards are conveyed to the pellet stockpile, which holds about 30,000 tons.
Pellet Loadout and Shipping
The pellets are now ready for shipping by train to customers or to ore docks. They are sent to blast furnaces and steel mills, where they will be turned into finished steel.
A trainload of iron ore pellets bound for the blast furnace
National Steel Pellet Company’s iron ore pellets have the following characteristics (FOB Mine):
Total Iron: 65.85% Silica (SiO2): 4.5% Lime (CaO): 0.68% Phosphorous: 0.010
Size: %+1/4” 96.5% (after tumble) Compression Strength: 560 pounds
from: http://www.steel.org
2009/05/06
TATA STEEL PLANS TO RESTRUCTURE CORUS'EUROPE OPERATIONS
By ceoaisra, Section AISRA NEWS
Posted on Mon May 04, 2009 at 03:50:31 AM EST
The global slump in steel prices and demand has prompted Tata Steel to consider restructuring the continental European operations of Corus, the Anglo-Dutch steel major it acquired in 2007 for $12 billion. The focus of the review is Corns' plants in Spain, France and the Netherlands, which could include selling these units if the need arises. Tata Steel's consolidated profits fell 48 per cent to Rs 732 crore in the quarter ended December, after Corus faced a drop in sales (the company does not give Corus' results separately). Corus, which has an annual production capacity of 20 million tonnes, has plants in the UK, the Netherlands, Germany, France and Belgium. This week, the company suffered a setback after an Italian buyer, Marcegaglia, backed out from a deal to buy its Teesside plant in northern England, saying the $480 million deal would financially stretch the company. An e-mail sent to the company spokesperson on Wednesday did not elicit a response. To limit losses, Corus has already decided to divest in downstream businesses. Briand Investments, an affiliate of UK-based investment group Klesch, had agreed to acquire Corus' aluminium smelters at Voerde in Germany and Delfcijl in the Netherlands. The company has also closed the three service centres in the UK. As part of restructuring its building systems division, it has closed units in south Wales and relocated the facilities to Shot-ton in north-east England. Tata Steel, which has about $9 billion debt in its books, is also looking to roll over the $4 billion of debt it raised to buy Corus. Sources said the company planned to extend the loan repayment period for three years to help Corus weather the downturn. Tata Steel has to repay $795 million in 2009-10 and $1.3 billion in 2010-11; however, the company is free from repayment until December 2009. "The company may use part its $1.9 billion reserves for the loan repayment, but that will adversely affect its expansion plans in India. The company requires $1.2 billion for its capital expenditure during this fiscal," tsaid a Mumbai-based analyst. Corus, the analyst added, has already cut 40 per cent of its production after the demand slump, so cash flows from Europe also will be lower. "The restricted cash flow will affect Corus' plans to acquire iron ore and coking coal mines for raw material security," said a Mumbai-based analyst.The Tata Steel and Corus managements have already gone through some top-level changes after Philippe Varin resigned as chief executive to head French-car maker PSA Peugeot Citroen. Kirby Adams, former chief executive of Blue Scope Steel in Australia, has succeeded Varin.
Posted on Mon May 04, 2009 at 03:50:31 AM EST
The global slump in steel prices and demand has prompted Tata Steel to consider restructuring the continental European operations of Corus, the Anglo-Dutch steel major it acquired in 2007 for $12 billion. The focus of the review is Corns' plants in Spain, France and the Netherlands, which could include selling these units if the need arises. Tata Steel's consolidated profits fell 48 per cent to Rs 732 crore in the quarter ended December, after Corus faced a drop in sales (the company does not give Corus' results separately). Corus, which has an annual production capacity of 20 million tonnes, has plants in the UK, the Netherlands, Germany, France and Belgium. This week, the company suffered a setback after an Italian buyer, Marcegaglia, backed out from a deal to buy its Teesside plant in northern England, saying the $480 million deal would financially stretch the company. An e-mail sent to the company spokesperson on Wednesday did not elicit a response. To limit losses, Corus has already decided to divest in downstream businesses. Briand Investments, an affiliate of UK-based investment group Klesch, had agreed to acquire Corus' aluminium smelters at Voerde in Germany and Delfcijl in the Netherlands. The company has also closed the three service centres in the UK. As part of restructuring its building systems division, it has closed units in south Wales and relocated the facilities to Shot-ton in north-east England. Tata Steel, which has about $9 billion debt in its books, is also looking to roll over the $4 billion of debt it raised to buy Corus. Sources said the company planned to extend the loan repayment period for three years to help Corus weather the downturn. Tata Steel has to repay $795 million in 2009-10 and $1.3 billion in 2010-11; however, the company is free from repayment until December 2009. "The company may use part its $1.9 billion reserves for the loan repayment, but that will adversely affect its expansion plans in India. The company requires $1.2 billion for its capital expenditure during this fiscal," tsaid a Mumbai-based analyst. Corus, the analyst added, has already cut 40 per cent of its production after the demand slump, so cash flows from Europe also will be lower. "The restricted cash flow will affect Corus' plans to acquire iron ore and coking coal mines for raw material security," said a Mumbai-based analyst.The Tata Steel and Corus managements have already gone through some top-level changes after Philippe Varin resigned as chief executive to head French-car maker PSA Peugeot Citroen. Kirby Adams, former chief executive of Blue Scope Steel in Australia, has succeeded Varin.
DEVELOPMENT OF HEMATITE BENEFICIATION AND PELLET TECHNOLOGY OF INDIA AND CHINA
By ceoaisra, Section TECHNOLOGY NEWS Posted on Tue Feb 05, 2008 at 01:46:02 AM EST
DEVELOPMENT OF HEMATITE BENEFICIATION AND PELLET TECHNOLOGY OF INDIA AND CHINA
Through the technology solution comparison of the hematite beneficiation and pellet between India and China in this paper, the conclusion can be summarized as below: China has rich experience in the technology of hematite beneficiation and pelletisation which can be completely applied in India hematite beneficiation and peilet industry and provide a platform for the cooperation of India and China's iron ore beneficiation and pellet technology.Keywords: beneficiation; pellet; hematite.
CHINA'S IRON ORE RESOURCE CONDITION
With fast and stable development of China's economy, the steel industry has been developing promptly. The requirement parameters of all the local steel companies have been increasing rapidly. We can't depend on only high quality ore resources from abroad just because the local ore mine can't meet the requirement far. According to the statistics, China imported iron ore more than 10 million tonnes in the year of 1985, while in 2002 more than l00 million tonnes, in 2004 more than 200 million tons, in 2006 more than 275 million tons. In the local iron ore resource, the easily to be beneficiated magnetite has become less and less. For the purpose of making best use of the local iron ore resource, enhance the degree of self-sufficiency of the local steel companies, reduce the ore importing pressure and keep in good supply of the high quality iron ore, we must promote the high efficiency development and utilization of the poor iron ore resource based on the science and technology improvement. In China the iron mine has a lot of deposits with complex deposit condition, ore types are too many, sulfur, phosphor and silicon dioxide etc. malign materials contents are high, multi component inter growth iron mine occupies a high ratio. Further more the useful components the inlet grain size are small, so the beneficiation with big difficulty, low rate and poor product quality. Due to poor quality of China's iron ore resource, causes the development of the iron ore beneficiation of China and the overall level is very high. Especially at recent years, we researched and successfully utilised the new advanced high efficiency beneficiation equipment, flotation reagent and new beneficiation process, so caused the beneficiation process index to get the breakthrough development.
THE BENEFICIATION PRACTICE OF CHINA'S HEMATITE
The hematite iron includes magnetite-hematite mixture ore, which is the important iron resource of China. In the beginning of 1960s, the main hematite processing technology with poor production index was baking-magnetic separation and single flotation. In recent years the hematite beneficiation got breakthrough because of the new process, new equipment and reagent are adopted.
ANGANG DIAO JUNTAI BENEFICIATION PLANT
The hematite is the only iron ore in the crude ore of AnGang Diao Juntai beneficiation plant, gangue is quartz, the crystallization grain of the mine is big. The beneficiation plant design capacity is 9MT/ year, adopted two sections continuous grinding mine, weak-separation--strong separation-- reverse flotation process. On the basis of iron grade 28 per cent, the beneficiation index reaches: the concentrate iron grade 67 per cent above, the iron recovery more than 75 per cent, tailing iron grade about 10 per cent.
ANGANG QIDASHAN BENEFICIATION PLANT
The hematite is the only iron ore in the crude ore of AnGang QiDaShan beneficiation plant, gangue is quartz, the crystallization grain of the mine is small. The beneficiation plant design capability is 8MT/annum. The original design adopted baking -- magnetic separation -- flotation process technology; the concentrate only can get the grade about 63 per cent. After the improvement of the process technology, adopted the process technology of stage grinding, rough concentrate rewashing and weak magnetic separation -- strong magnetic separation-- reverse flotation, so that on the base of crude iron ore 29 per cent can get the index of concentrate grade more than 67.5 per cent, the recovery rate higher than 70 per cent and the tailing iron grade about 13 per cent.
ANGANG HUJIAMIAO BENEFICIATION PLANT
The hematite is the only iron ore in the crude ore of AnGang HuJiaMiao beneficiation plant, gangue is quartz, the crystallization grain of the mine is asymmetry. The beneficiation plant design capability is 8MT/annum, adopted the process technology of stage grinding, rough and fine separation, weak magnetic separation -- strong magnetic separation-- gravity separation -- reverse flotation, so that on the base of crude iron ore 28 per cent can get the index of concentrate grade more than 67 per cent, the recovery rate higher than 70 per cent and the tailing iron grade about 11 per cent.
WUYANG IRON MINE TIESHANMIAO BENEFICITION PLANT
The hematite is the only iron ore in the crude ore of WuYang iron mine TieShanMiao beneficiation plant, gangue is quartz, the crystallization grain of the mine is asymmetry. The beneficiation plant design capability is 3.5MT/annum, adopted the process technology of stage grinding, weak magnetic separation--strong magnetic separation--gravity separation and reverse flotation for the tailing of gravity separation, so that on the base of crude iron ore 29 per cent can get the beneficiation index of concentrate grade more than 65 per cent, the recovery rate higher than 70 per cent and the tailing iron grade about 12 per cent.
TANGSHAN SIJIAYING BENEFICIATION PLANT
The hematite is the only iron ore in the crude ore of Sijiaying beneficiation plant, gangue is quartz, the crystallization grain of the mine is asymmetry. The beneficiation plant design capability is 7MT/annum, adopted the process technology of stage grinding, respective separation for rough and fine ore, gravity separation--magnetic separation--reverse flotation, so that on the base of crude iron ore grade 29 per cent can get the beneficiation index of concentrate grade more than 66.55 per cent, the recovery rate higher than 75 per cent and the tailing iron grade about 10 per cent.
CHINA'S IRON ORE PELLET TECHNOLOGY
Though the starting of China's iron ore pellet technology is late, the development is fast .In the beginning of 1990s, there were only several shaft furnace pellet plant with several million tonnes output, while till 2007 develops into more than 20 production plants and the output exceeds 100 million tons, the production equipment changed from the low efficient shaft furnace into belt baking machine and traveling grate machine-rotary kiln, the single equipment capability reaches 5MT/annum. The pellet plant newly put into production almost adopt imported iron ore with the main content hematite which is more than 80 per cent of the raw material. Since the magnetite is the main part of the iron concentrate in local, almost all producers blended the local magnetite during the pellet production. At present the ongoing WISCO No.2 pellet production line, and Bao Steel ZhanJiang pellet plant both adopt the hematite as the only iron ore material, the designed capacity is 5MT/annum. Almost all pellet plants of China adopt traveling grate machine-rotary kiln except a few who adopt shaft furnace and belt type baking machine. The advantages are saving the building investment, stable product quality, strong adaptability for the fuel, natural gas, oil and coal all can be used.
PROFILE OF INDIAN IRON MINE
India is one of the countries with the most abundant mineral reserves in the world. The basic reserve of the iron ore is about 23.58 million tonnes, of which about 12.9 million tonnes of hematite and 10.68 million tonnes of magnetite ore, ranking sixth in the world and accounting for about 6.8-7 per cent of the world's total reserves. At present, the output of iron ore India ranks eighth accounting for about 8 per cent of the world's total reserves. As the demands of domestic and export boosted in recent years, the iron ore output of India increased substantially. The annual output was 86.22million tonnes in 2002, and reached 145.94 million tonnes in 2005, of which 48.15 million tonnes for domestic demand and 78.14 million tonnes for export. Currently India's total iron ore mining capacity is 154.43 million tonnes annually, of which state-owned enterprises account for 51 per cent and the rest is produced by private enterprises. The lump ore that contains over 65 per cent iron accounts for 21 per cent of India's total iron ore output, and the iron ore fines that contains over 65 per cent iron accounted for 16.2 percent. The Indian government divides the resource into three grades according to the iron level: over 65 per cent is high-grade ore, 62-65 per cent is mid-grade ore, and less than 62 percent is low-grade ore. Of the hematite resources, 10.4 per cent are high-grade ore that concentrate in Chattisgarh, Orissa, Karnataka and Jharkhand; and 34 per cent are mid-grade ore that concentrate in Orissa, Karnataka, Jharkhand and Madhya Pradesh. Since many Indian iron ore resources are located in the nature reserves or in the jungles and mountains, it's difficult to exploit, so in the 23.58 billion tons of iron ore reserves of India the basic reserves that can be exploited is only 17.27 billion tonnes. Environmental problem has become a key issue affecting the future of India's iron ore resources exploitation. Such as the mine owned by Kudremukh Iron Ore Company (KIOCL) in the forest land of West Coast in Karnataka, has ever been India's largest single mine. The output of the raw ore reached 20 million tonnes annually, and its pellet ore also reached 350,000 tonnes every year, but because of the opposition of the domestic environmental organisations, the Indian High Court adjudged to close the mine at the end of December 2005.
INDIAN IRON ORE BENEFICIATION AND PELLET
Since the Indian iron resource almost is the rich ore with high iron grade, the grade more than 60 per cent iron ore occupies more than a half of the total. The already exploited mines have few beneficiation process and only a few mine add the washing process in ore crushing screening procedure, and leave the slime as tailings impoundment. The main raw material of the pellet is the powder of the rich ore, which utilizes concentrate from beneficiated ore. Under the present scenario with the stable growing of iron ore price, the question how Lo increase the utilisation rate of the iron resource and protect the environment of the mine has been the priority by the government and enterprises. On one hand, the slime obtained from the washing are comprehensively to be used, reduce the tailing impoundment and reduce the pollution for the air; On the other hand, beneficiate the powder which obtained from the lump ore production to reduce the silicon and alumina etc, gangue volume and improve the quality. Compared to China's hematite, Indian hematite has absolute priority in the grade quality. The grade of China's hematite crude ore is only about 30 per cent, while that of Indian ore can reach 45 per cent and above. Mono mineral crystallization grain of China's hematite is relatively coarse and the gangues are mainly quartz, the ore with high hardness is difficult to be slimed, so the separation rate is high. In the low-grade hematite of India, the grain of iron ore and gangue are both small and the gangue contents a large amount of alumino-silicate except, so the beneficiation should decrease silicon as well as aluminum. The sulphur and phosphor quantity contained in Indian hematite are also relatively high, so it's difficult for beneficiation. The hardness of the India's low grade hematite is low, which gives advantage of low energy consumption when crushing and grinding, while the disadvantage is the ore easily to be slimed and after sliming the ore can be washed away with water which cause low recovery of iron. Borrowing experience from the beneficiation of China, take beneficiation into the low-grade hematite of India and further utilize the concentrate beneficiated for pellet production has already got big improvement. Utilizing the high microseism gradient magnetic separator and combine the reverse notation process, can get concentrate grade about 66 per cent, SiO2, less than 4 per cent, A12O3 less than 3 per cent, the recovery more than 65 per cent as well the sulphur and phosphor content volume can be reduced. Recently the hematite pellet test of India got success and the pellet quality reaches the requirement of blast furnace iron making. Technically it's not a problem to use the concentrate from the low-grade hematite beneficiation for the pellet production.
CONCLUSION
From the comparison of hematite resource condition, ore property, beneficiation type index and the pellet technology between China and India, though the big difference exists in the quantity, quality and beneficiation type index, the rich experience of China in hematite beneficiation and pellet technology can be completely utilized in hematite beneficiation and pellet technology of India. The successful test and research in partial mine validate this point and will provide the reference for the cooperation of China and India in the beneficiation and pellet technology area.
Source by: Joint Plant Committee
DEVELOPMENT OF HEMATITE BENEFICIATION AND PELLET TECHNOLOGY OF INDIA AND CHINA
Through the technology solution comparison of the hematite beneficiation and pellet between India and China in this paper, the conclusion can be summarized as below: China has rich experience in the technology of hematite beneficiation and pelletisation which can be completely applied in India hematite beneficiation and peilet industry and provide a platform for the cooperation of India and China's iron ore beneficiation and pellet technology.Keywords: beneficiation; pellet; hematite.
CHINA'S IRON ORE RESOURCE CONDITION
With fast and stable development of China's economy, the steel industry has been developing promptly. The requirement parameters of all the local steel companies have been increasing rapidly. We can't depend on only high quality ore resources from abroad just because the local ore mine can't meet the requirement far. According to the statistics, China imported iron ore more than 10 million tonnes in the year of 1985, while in 2002 more than l00 million tonnes, in 2004 more than 200 million tons, in 2006 more than 275 million tons. In the local iron ore resource, the easily to be beneficiated magnetite has become less and less. For the purpose of making best use of the local iron ore resource, enhance the degree of self-sufficiency of the local steel companies, reduce the ore importing pressure and keep in good supply of the high quality iron ore, we must promote the high efficiency development and utilization of the poor iron ore resource based on the science and technology improvement. In China the iron mine has a lot of deposits with complex deposit condition, ore types are too many, sulfur, phosphor and silicon dioxide etc. malign materials contents are high, multi component inter growth iron mine occupies a high ratio. Further more the useful components the inlet grain size are small, so the beneficiation with big difficulty, low rate and poor product quality. Due to poor quality of China's iron ore resource, causes the development of the iron ore beneficiation of China and the overall level is very high. Especially at recent years, we researched and successfully utilised the new advanced high efficiency beneficiation equipment, flotation reagent and new beneficiation process, so caused the beneficiation process index to get the breakthrough development.
THE BENEFICIATION PRACTICE OF CHINA'S HEMATITE
The hematite iron includes magnetite-hematite mixture ore, which is the important iron resource of China. In the beginning of 1960s, the main hematite processing technology with poor production index was baking-magnetic separation and single flotation. In recent years the hematite beneficiation got breakthrough because of the new process, new equipment and reagent are adopted.
ANGANG DIAO JUNTAI BENEFICIATION PLANT
The hematite is the only iron ore in the crude ore of AnGang Diao Juntai beneficiation plant, gangue is quartz, the crystallization grain of the mine is big. The beneficiation plant design capacity is 9MT/ year, adopted two sections continuous grinding mine, weak-separation--strong separation-- reverse flotation process. On the basis of iron grade 28 per cent, the beneficiation index reaches: the concentrate iron grade 67 per cent above, the iron recovery more than 75 per cent, tailing iron grade about 10 per cent.
ANGANG QIDASHAN BENEFICIATION PLANT
The hematite is the only iron ore in the crude ore of AnGang QiDaShan beneficiation plant, gangue is quartz, the crystallization grain of the mine is small. The beneficiation plant design capability is 8MT/annum. The original design adopted baking -- magnetic separation -- flotation process technology; the concentrate only can get the grade about 63 per cent. After the improvement of the process technology, adopted the process technology of stage grinding, rough concentrate rewashing and weak magnetic separation -- strong magnetic separation-- reverse flotation, so that on the base of crude iron ore 29 per cent can get the index of concentrate grade more than 67.5 per cent, the recovery rate higher than 70 per cent and the tailing iron grade about 13 per cent.
ANGANG HUJIAMIAO BENEFICIATION PLANT
The hematite is the only iron ore in the crude ore of AnGang HuJiaMiao beneficiation plant, gangue is quartz, the crystallization grain of the mine is asymmetry. The beneficiation plant design capability is 8MT/annum, adopted the process technology of stage grinding, rough and fine separation, weak magnetic separation -- strong magnetic separation-- gravity separation -- reverse flotation, so that on the base of crude iron ore 28 per cent can get the index of concentrate grade more than 67 per cent, the recovery rate higher than 70 per cent and the tailing iron grade about 11 per cent.
WUYANG IRON MINE TIESHANMIAO BENEFICITION PLANT
The hematite is the only iron ore in the crude ore of WuYang iron mine TieShanMiao beneficiation plant, gangue is quartz, the crystallization grain of the mine is asymmetry. The beneficiation plant design capability is 3.5MT/annum, adopted the process technology of stage grinding, weak magnetic separation--strong magnetic separation--gravity separation and reverse flotation for the tailing of gravity separation, so that on the base of crude iron ore 29 per cent can get the beneficiation index of concentrate grade more than 65 per cent, the recovery rate higher than 70 per cent and the tailing iron grade about 12 per cent.
TANGSHAN SIJIAYING BENEFICIATION PLANT
The hematite is the only iron ore in the crude ore of Sijiaying beneficiation plant, gangue is quartz, the crystallization grain of the mine is asymmetry. The beneficiation plant design capability is 7MT/annum, adopted the process technology of stage grinding, respective separation for rough and fine ore, gravity separation--magnetic separation--reverse flotation, so that on the base of crude iron ore grade 29 per cent can get the beneficiation index of concentrate grade more than 66.55 per cent, the recovery rate higher than 75 per cent and the tailing iron grade about 10 per cent.
CHINA'S IRON ORE PELLET TECHNOLOGY
Though the starting of China's iron ore pellet technology is late, the development is fast .In the beginning of 1990s, there were only several shaft furnace pellet plant with several million tonnes output, while till 2007 develops into more than 20 production plants and the output exceeds 100 million tons, the production equipment changed from the low efficient shaft furnace into belt baking machine and traveling grate machine-rotary kiln, the single equipment capability reaches 5MT/annum. The pellet plant newly put into production almost adopt imported iron ore with the main content hematite which is more than 80 per cent of the raw material. Since the magnetite is the main part of the iron concentrate in local, almost all producers blended the local magnetite during the pellet production. At present the ongoing WISCO No.2 pellet production line, and Bao Steel ZhanJiang pellet plant both adopt the hematite as the only iron ore material, the designed capacity is 5MT/annum. Almost all pellet plants of China adopt traveling grate machine-rotary kiln except a few who adopt shaft furnace and belt type baking machine. The advantages are saving the building investment, stable product quality, strong adaptability for the fuel, natural gas, oil and coal all can be used.
PROFILE OF INDIAN IRON MINE
India is one of the countries with the most abundant mineral reserves in the world. The basic reserve of the iron ore is about 23.58 million tonnes, of which about 12.9 million tonnes of hematite and 10.68 million tonnes of magnetite ore, ranking sixth in the world and accounting for about 6.8-7 per cent of the world's total reserves. At present, the output of iron ore India ranks eighth accounting for about 8 per cent of the world's total reserves. As the demands of domestic and export boosted in recent years, the iron ore output of India increased substantially. The annual output was 86.22million tonnes in 2002, and reached 145.94 million tonnes in 2005, of which 48.15 million tonnes for domestic demand and 78.14 million tonnes for export. Currently India's total iron ore mining capacity is 154.43 million tonnes annually, of which state-owned enterprises account for 51 per cent and the rest is produced by private enterprises. The lump ore that contains over 65 per cent iron accounts for 21 per cent of India's total iron ore output, and the iron ore fines that contains over 65 per cent iron accounted for 16.2 percent. The Indian government divides the resource into three grades according to the iron level: over 65 per cent is high-grade ore, 62-65 per cent is mid-grade ore, and less than 62 percent is low-grade ore. Of the hematite resources, 10.4 per cent are high-grade ore that concentrate in Chattisgarh, Orissa, Karnataka and Jharkhand; and 34 per cent are mid-grade ore that concentrate in Orissa, Karnataka, Jharkhand and Madhya Pradesh. Since many Indian iron ore resources are located in the nature reserves or in the jungles and mountains, it's difficult to exploit, so in the 23.58 billion tons of iron ore reserves of India the basic reserves that can be exploited is only 17.27 billion tonnes. Environmental problem has become a key issue affecting the future of India's iron ore resources exploitation. Such as the mine owned by Kudremukh Iron Ore Company (KIOCL) in the forest land of West Coast in Karnataka, has ever been India's largest single mine. The output of the raw ore reached 20 million tonnes annually, and its pellet ore also reached 350,000 tonnes every year, but because of the opposition of the domestic environmental organisations, the Indian High Court adjudged to close the mine at the end of December 2005.
INDIAN IRON ORE BENEFICIATION AND PELLET
Since the Indian iron resource almost is the rich ore with high iron grade, the grade more than 60 per cent iron ore occupies more than a half of the total. The already exploited mines have few beneficiation process and only a few mine add the washing process in ore crushing screening procedure, and leave the slime as tailings impoundment. The main raw material of the pellet is the powder of the rich ore, which utilizes concentrate from beneficiated ore. Under the present scenario with the stable growing of iron ore price, the question how Lo increase the utilisation rate of the iron resource and protect the environment of the mine has been the priority by the government and enterprises. On one hand, the slime obtained from the washing are comprehensively to be used, reduce the tailing impoundment and reduce the pollution for the air; On the other hand, beneficiate the powder which obtained from the lump ore production to reduce the silicon and alumina etc, gangue volume and improve the quality. Compared to China's hematite, Indian hematite has absolute priority in the grade quality. The grade of China's hematite crude ore is only about 30 per cent, while that of Indian ore can reach 45 per cent and above. Mono mineral crystallization grain of China's hematite is relatively coarse and the gangues are mainly quartz, the ore with high hardness is difficult to be slimed, so the separation rate is high. In the low-grade hematite of India, the grain of iron ore and gangue are both small and the gangue contents a large amount of alumino-silicate except, so the beneficiation should decrease silicon as well as aluminum. The sulphur and phosphor quantity contained in Indian hematite are also relatively high, so it's difficult for beneficiation. The hardness of the India's low grade hematite is low, which gives advantage of low energy consumption when crushing and grinding, while the disadvantage is the ore easily to be slimed and after sliming the ore can be washed away with water which cause low recovery of iron. Borrowing experience from the beneficiation of China, take beneficiation into the low-grade hematite of India and further utilize the concentrate beneficiated for pellet production has already got big improvement. Utilizing the high microseism gradient magnetic separator and combine the reverse notation process, can get concentrate grade about 66 per cent, SiO2, less than 4 per cent, A12O3 less than 3 per cent, the recovery more than 65 per cent as well the sulphur and phosphor content volume can be reduced. Recently the hematite pellet test of India got success and the pellet quality reaches the requirement of blast furnace iron making. Technically it's not a problem to use the concentrate from the low-grade hematite beneficiation for the pellet production.
CONCLUSION
From the comparison of hematite resource condition, ore property, beneficiation type index and the pellet technology between China and India, though the big difference exists in the quantity, quality and beneficiation type index, the rich experience of China in hematite beneficiation and pellet technology can be completely utilized in hematite beneficiation and pellet technology of India. The successful test and research in partial mine validate this point and will provide the reference for the cooperation of China and India in the beneficiation and pellet technology area.
Source by: Joint Plant Committee
New gasification method to produce Direct Reduced Iron (DRI)
A small Dutch company developed a new gasification concept to produce syngas in order to reduce iron ore in a direct reduction process using pulsed combustion burner technology. Utilising a gasifier to generate reducing gases is a technically and commercially viable method for innovative steelmakers to produce DRI in areas where low cost natural gas is not available. The company is looking for a commercial agreement with a company that can use the technology in its product.
Commercial gasifiers have been in operation for over 50 years. In general, gasification can be defined as a partial oxidation process in which carbonaceous fuel (gas, liquid, or solid) reacts at high temperature and usually at high pressure with oxygen and possible steam to produce a synthesis gas. This gas can then be used to fuel a Direct Reduction Process. Utilising a gasifier to generate reducing gases is a technically and commercially viable method for innovative steelmakers to produce DRI in areas where low cost natural gas is not available. Even better economics can be derived when the project includes a melt shop and an integrated gasification combined cycle-based power plant.
Pulsed combustion burners can be used wherever you use conventional burners. In the last decade the development has made progress, resulting in a number of projects around the world utilising the above principle with great advantages, like e.g. high production increase in case of drying applications. The company is capable of building stable industrial high-frequency pulsed high-power burners. Pulsed combustion has been known for a long time. In the last decade the development has made further progress, resulting in a number of projects around the world utilising the above principle. At this moment the company has built a gasification unit of several MW using the same principle of pulsed combustion.
During the development a number of obstacles have been encountered and overcome, namely the ability to up scale, and to increase frequency proved to be a stumbling block for the growth of this technology and its application. However this did not prevent a number of diverse applications. A few examples are:
- gasification units,
- product drying such as vegetables,
- chemical powder drying with small particles,
- the ability to utilise various waste materials for burning,
- typical sludge incineration from sewage plants and industrial water waste,
- boiler, heaters of all kind,
- desalination plants (for evaporators) to produce fresh water installations,
- air heaters,
- incinerators,
- production of steam for turbines and electricity generation,
- kilns. Innovative Aspects: The company also claims to have perfected gasification and burner technology where it would be possible to design and build a 10 MW burner, where further up scaling would not present any problem.
This technology is unique in so far that with this technology the company is capable of designing and manufacturing this type of pulsating burner without any moving parts, resulting in an industrial stable high-frequency high-power burner and/or gasification unit. Main Advantages: - High efficient gasification.
- To have an efficient burning rate resulting in a lower energy cost.
- By having a higher energy transfer rate will mean smaller and cost-effective equipment (fast return of investment).
- Creating lower pollution (low NOx in accordance with the recent Kyoto environmental requirements).
- In the application of sludge, a significant reduction of possible precipitor agent such as polyelectrolyte commonly used in the de-watering processes.
- It has a built in self-cleaning advantage, due to the pulsating mechanism of the combustion technology.
- The final product after drying tends to have a light structure which a high porosity.
from:http://www.invenia.es/tech:06_nl_nlse_0f8l
Commercial gasifiers have been in operation for over 50 years. In general, gasification can be defined as a partial oxidation process in which carbonaceous fuel (gas, liquid, or solid) reacts at high temperature and usually at high pressure with oxygen and possible steam to produce a synthesis gas. This gas can then be used to fuel a Direct Reduction Process. Utilising a gasifier to generate reducing gases is a technically and commercially viable method for innovative steelmakers to produce DRI in areas where low cost natural gas is not available. Even better economics can be derived when the project includes a melt shop and an integrated gasification combined cycle-based power plant.
Pulsed combustion burners can be used wherever you use conventional burners. In the last decade the development has made progress, resulting in a number of projects around the world utilising the above principle with great advantages, like e.g. high production increase in case of drying applications. The company is capable of building stable industrial high-frequency pulsed high-power burners. Pulsed combustion has been known for a long time. In the last decade the development has made further progress, resulting in a number of projects around the world utilising the above principle. At this moment the company has built a gasification unit of several MW using the same principle of pulsed combustion.
During the development a number of obstacles have been encountered and overcome, namely the ability to up scale, and to increase frequency proved to be a stumbling block for the growth of this technology and its application. However this did not prevent a number of diverse applications. A few examples are:
- gasification units,
- product drying such as vegetables,
- chemical powder drying with small particles,
- the ability to utilise various waste materials for burning,
- typical sludge incineration from sewage plants and industrial water waste,
- boiler, heaters of all kind,
- desalination plants (for evaporators) to produce fresh water installations,
- air heaters,
- incinerators,
- production of steam for turbines and electricity generation,
- kilns. Innovative Aspects: The company also claims to have perfected gasification and burner technology where it would be possible to design and build a 10 MW burner, where further up scaling would not present any problem.
This technology is unique in so far that with this technology the company is capable of designing and manufacturing this type of pulsating burner without any moving parts, resulting in an industrial stable high-frequency high-power burner and/or gasification unit. Main Advantages: - High efficient gasification.
- To have an efficient burning rate resulting in a lower energy cost.
- By having a higher energy transfer rate will mean smaller and cost-effective equipment (fast return of investment).
- Creating lower pollution (low NOx in accordance with the recent Kyoto environmental requirements).
- In the application of sludge, a significant reduction of possible precipitor agent such as polyelectrolyte commonly used in the de-watering processes.
- It has a built in self-cleaning advantage, due to the pulsating mechanism of the combustion technology.
- The final product after drying tends to have a light structure which a high porosity.
from:http://www.invenia.es/tech:06_nl_nlse_0f8l
2009/05/05
Direct reduced iron
Direct-reduced iron (DRI), also called sponge iron [1], is produced from direct reduction of iron ore (in the form of lumps, pellets or fines) by a reducing gas produced from natural gas or coal. The reducing gas is a mixture majority of Hydrogen (H2) and Carbon Monoxide (CO) which acts as reducing agent. This process of directly reducing the iron ore in solid form by reducing gases is called direct reduction.
The conventional route for making steel consists of sintering or pelletization plants, coke ovens, blast furnaces, and basic oxygen furnaces. Such plants require high capital expenses and raw materials of stringent specifications. Coking coal is needed to make a coke strong enough to support the burden in the blast furnace. Integrated steel plants of less than one million tons annual capacity are generally not economically viable. The coke ovens and sintering plants in an integrated steel plant are polluting and expensive units.
Process
Direct reduction, is an alternative route of iron making, has been developed to overcome some of these difficulties of conventional blast furnaces. DRI is successfully manufactured in various parts of the world through either gas or coal-based technology. Iron ore is reduced in solid state at 800 —1050°C either by reducing gas (H2+CO) or coal. The specific investment and operating costs of direct reduction plants are low compared to integrated steel plants, and are more suitable for many developing countries where supplies of coking coal are limited.
This statement is incorrect - the blast furnace has lower operating costs. The direct reduction process is intrinsically more energy efficient than the blast furnace because it operates at a lower temperature, and there are several other factors which make it economical:
Direct-reduced iron is richer in iron than pig iron, typically 90-94% total iron (depending on the quality of the raw ore) as opposed to about 93% for molten pig iron, and an excellent feedstock for the electric furnaces used by mini mills, allowing them to use lower grades of scrap for the rest of the charge or to produce higher grades of steel.
Hot-briquetted iron (HBI) is a compacted form of DRI designed for ease of shipping, handling, and storage.
Hot Direct Reduced Iron (HDRI) is iron not cooled before discharge from the reduction furnace, immediately transported to a waiting electric arc furnace and charged thereby saving energy.
The direct reduction process uses pelletized iron ore or natural 'lump' ore. One exception is the fluidized bed process which uses (requires) sized iron ore particles. Select few ores are suitable for direct reduction.
The direct reduction process can use natural gas contaminated with inert gases, avoiding the need to remove these gases for other use. However, any inert gas contamination of the reducing gas lowers the effect (quality) of that gas stream and the thermal efficiency of the process.
Supplies of powdered ore and raw natural gas are both available in areas such as Northern Australia, avoiding transport costs for the gas. In most cases the DRI plant is located near natural gas source as it is more cost effective to ship the ore rather than the gas.
India is the world’s largest producer of direct-reduced iron, a vital constituent of the steel industry. Many other countries use variants of the process, so providing iron for local engineering industries.
The conventional route for making steel consists of sintering or pelletization plants, coke ovens, blast furnaces, and basic oxygen furnaces. Such plants require high capital expenses and raw materials of stringent specifications. Coking coal is needed to make a coke strong enough to support the burden in the blast furnace. Integrated steel plants of less than one million tons annual capacity are generally not economically viable. The coke ovens and sintering plants in an integrated steel plant are polluting and expensive units.
Process
Direct reduction, is an alternative route of iron making, has been developed to overcome some of these difficulties of conventional blast furnaces. DRI is successfully manufactured in various parts of the world through either gas or coal-based technology. Iron ore is reduced in solid state at 800 —1050°C either by reducing gas (H2+CO) or coal. The specific investment and operating costs of direct reduction plants are low compared to integrated steel plants, and are more suitable for many developing countries where supplies of coking coal are limited.
This statement is incorrect - the blast furnace has lower operating costs. The direct reduction process is intrinsically more energy efficient than the blast furnace because it operates at a lower temperature, and there are several other factors which make it economical:
Direct-reduced iron is richer in iron than pig iron, typically 90-94% total iron (depending on the quality of the raw ore) as opposed to about 93% for molten pig iron, and an excellent feedstock for the electric furnaces used by mini mills, allowing them to use lower grades of scrap for the rest of the charge or to produce higher grades of steel.
Hot-briquetted iron (HBI) is a compacted form of DRI designed for ease of shipping, handling, and storage.
Hot Direct Reduced Iron (HDRI) is iron not cooled before discharge from the reduction furnace, immediately transported to a waiting electric arc furnace and charged thereby saving energy.
The direct reduction process uses pelletized iron ore or natural 'lump' ore. One exception is the fluidized bed process which uses (requires) sized iron ore particles. Select few ores are suitable for direct reduction.
The direct reduction process can use natural gas contaminated with inert gases, avoiding the need to remove these gases for other use. However, any inert gas contamination of the reducing gas lowers the effect (quality) of that gas stream and the thermal efficiency of the process.
Supplies of powdered ore and raw natural gas are both available in areas such as Northern Australia, avoiding transport costs for the gas. In most cases the DRI plant is located near natural gas source as it is more cost effective to ship the ore rather than the gas.
India is the world’s largest producer of direct-reduced iron, a vital constituent of the steel industry. Many other countries use variants of the process, so providing iron for local engineering industries.
beneficiation of IRON ORE
Effectiveness Of Gravity Concentration For The Beneficiation Of Itakpe (Nigeria) Iron Ore Achieved Through Jigging Operation.
P. A. OLUBAMBI 1,2* and J. H. POTGIETER
Department of Metallurgical and Materials Engineering, Federal University of Technology, Akure, Nigeria2School of Process and Materials Engineering, University of the Witwatersrand, Johannesburg, South Africa
Abstract:
This study investigates the1 effectiveness of gravity concentration for the beneficiation of Itakpe (Nigeria) iron ore achieved through jigging operations. Iron ore obtained from Itapke Iron Ore Mining Project, Kogi State, Nigeria which contains a very high amount of quartz as revealed by x-ray diffraction was crushed using the laboratory dodge crusher and ground in a laboratory ball mill. Particle size analysis was carried out over the range of +4750µm and -75µm in 12 different mesh sizes, and the ore was jigged in a Laboratory Denver Mineral Jig. The operating variables used to determine the recovery effectiveness of jigging include; particle size, dilution ratio and bedding thickness. Recovery of iron ore was assessed by determining the percentages of Fe in the underflow and overflow using the Atomic Absorption spectrometry (AAS) method. Optimum iron ore recovery of 71% was achieved when the jig was operated at medium stroke and speed, with a feed slurry of average dilution and at a particle size of 600µm.
Keywords: Iron ore; Jigging; Particle size; Dilution ratio; Bedding thickness.
INTRODUCTION
The Itakpe iron ore deposit has a reserve of about 200 million tonnes with an average iron ore content of 36%. This has to be beneficiated at a rate of 8 million tonnes per year to produce 64% Fe concentrate as sinter material for the Ajaoukuta blast furnace and 68% Fe concentrate as pellet feed for the direct reduction plant at Aladja, all in Nigeria. At this production rate, large quantities of tailings are obtained as waste product of the beneficiated iron ore (Adepoju and Olaleye 2001).
Since the goal of every mineral processing operation/technique is to effectively separate the valuable material from the gangue with minimum metal loss in tailings, the need to develop and employ a sustainable, effective and relatively economical method of separation is imperative. The concentration of the valuable minerals from the gangue involves exploitation of the differences in the mineral properties of the ore after effective comminution (Akande and Olaleye 2000).
Magnetic separation and flotation are the most widely accepted technologies for the upgrading of iron ore particles, but these processes result in iron concentrate with high amounts of very fine and/or interlocked silica particles (Yang, et al 2003). Mohanty (2002) also found that the ability of flotation to treat mixed-phase (middling) and weakly hydrophobic particles is not satisfactory. To address the aforementioned problems and thus to achieve a higher iron ore recovery, several new attempts and technologies are being developed with an added aim of achieving economical and environmental benefits through use of the jigging method (Parkinson, 1989; Yang, 1996; Honaker, et al, 1996; Galvin et al 2002).
The mineralogical characterization of Itakpe iron ore shows that it contains mainly hematite, magnetite and quartz whose specific gravities give sound basis for adopting the gravity concentration technique. In this study, the effectiveness of gravity techniques for concentrating iron ore from bulk Itakpe (Nigeria) iron ore was studied using the jigging method and the effects of operating variables on the recovery of iron ore using the laboratory Denver Mineral Jig were investigated.
MATERIALS AND METHODS
The bulk ore used in this study was obtained from the Itakpe mine, north of Okene in Kogi State, Middle Belt Region of Nigeria. Its chemical composition as revealed by X-ray fluorescence is as shown in Table1
The bulk ore used in this study was obtained from the Itakpe mine, north of Okene in Kogi State, Middle Belt Region of Nigeria.
The ore was broken into sizes that could be fed into the jaw crusher using a sledgehammer. Crushing was carried out in a laboratory dodge crusher and ground in a laboratory ball mill. Ore sieving was carried out using the laboratory sieve shaker as described by Pryor (1965) and Adepoju & Olaleye (2001) by placing 6000g of the ore in the uppermost ASTM standard sieve. The nest of the ASTM sieves was loaded with the ore and allowed to vibrate for 5 minutes. After the required time, the nest of sieves was taken apart and the amount of material retained on each sieve was weighed. Composition was determined by X-ray fluorescence.
100 grams of the ore of the same size from the product of the sieve analysis was stored in a tray to form the feed or head material for the jigging operation. Steel balls were spread to form a layer on the screen of the mineral jig as a bedding material to varying depths. The spigot of the hutch compartment was plugged with rubber cork and water was added to cover the ragging in the feeding compartment. The head or feed was fed into the feeding compartment.
Feed material mixed with water at varying dilutions was added to the jig and the jigging process was allowed for 4 minutes. At the end of each jigging operation, the spigot of the hutch compartment was opened and the product was collected as the underflow. The overflow materials left in the feeding compartment were scooped and washed out. The two products (underflow and overflow) were dried, weighed and recorded. The experiments were repeated with varying bedding thickness, dilution rates and particle sizes.
The amount of iron ore in each of the underflow and overflows were evaluated by determining the percentages of Fe in the samples using Atomic Absorption Spectrometry (AAS) method. A sample weight of 2g was the standard measurement for this experiment. Samples were dried prior to analysis. Samples were well mixed before weighing to make sure they were homogenous. The samples were digested with 20 ml of .01 M Hydrochloric acid (HCL) solution by shaking in plastic centrifuge tubes for 15-20 minutes. Concentration of each of the samples was measured against standard solutions. The composition of iron and silicon in each sample was then ascertained using a Buck Scientific model 200 atomic absorption spectrophotometer with air-acetylene flame.
RESULTS AND DISCUSSION
The results obtained from the particle size analysis are as shown in Table 2 and the compositional analyses revealed by X-ray are graphically presented in Figures 1, 2 and 3. Tables 3, 4, and 5 show the results obtained from the jigging operations and they are as graphically illustrated in Figures 4, 5 and 6.
Size analysis
It can be observed from Table 2 that the smaller the aperture of the sieve, the lower the weight% of Itakpe iron ore retained. The aperture range of 1180µm has the most retained weight% followed by 850µm and then 2000µm respectively. It was also observed in Table 2 that 2000µm has the most retained quantity of quartz followed by 1180µm then 350µm. The rate of reduction of both iron ore and quartz varies (Figures 1 to 3). Quartz dissipates easily, breaking down to fines with little applied stress. Though the hardness value of quartz (i.e. 7 on the Mohr’s scale) is a bit higher than the iron ore (Hematite (5.5-6.5), Magnetite (5.5-6), it is far more brittle than iron ore (Gribble, 1988).
The disparity in the cumulative weight% retained and cumulative passing of the ore is demonstrated by Figures 1 and 2. From both graphs, it can be seen that the cumulative weight% retained and cumulative passing graphs are inversely proportional to each other. The Gate-Gaudin-Schumann’s representation of sieve analysis in Figure 3 shows that the compositional distributions of the materials are linearly and uniformly distributed over a wide size range.
Separation effectiveness:
The results, as shown in Tables 4, 5, and 6 and Figures 4, 5 and 6, revealed that iron ore concentrate (underflow) was effectively separated from the tailings (overflow), which are essentially quartz. Theoretically, effective separation was possible because the quotient of the difference in their specific gravities is greater than 2.5 (Equation 1)
Effects of bedding thickness
The effects of bedding material and thickness of the bed on the recovery of iron ore are shown in Figures 4, 5 and 6. The bedding material and the thickness of the bedding materials used has an effect on the %Fe recovered as shown in Figure 6. Thick bedding causes more friction action during the suction stage, making concentration less possible. Thin bedding reduces friction during suction and also, allows easy passage of the Fe concentrate during jigging.
Effects of Particle size:
The effect of the particle size on the recovery of iron ore is also shown in Tables 4,5, and 6, and Figures 4, 5 and 6. The highest recovery was achieved at 600µm. Lower separation efficiency in finer particles is believed to be caused by the negligible mass associated with this size particles. Particles so small, that settle in accordance with Stroke’s law, are unsuitable for concentration (Wills, 1989). Lower recovery of Fe at larger particle sizes may be due to the reduced possibilities of the larger particles passing through the jig screen. A coarser particle will have a reduced chance of passing through the jig screen and thereby will report to the overflow as tailings (Mohanty, et al 2002).
CONCLUSION
The results of this project work have clearly shown that effective separation of iron ore concentrate from bulk Itakpe iron ore by jigging operations is possible. It is clearly revealed that the effectiveness of the separation was greatly influenced by the operating variables of the jig and the particle size of the ore. The result of the work has also shown that the optimum Fe recovery could be achieved when the jig is operated at medium stroke and speed under the conditions of a thin bed with feed slurry of average dilution at a particle size range of 600µm.
P. A. OLUBAMBI 1,2* and J. H. POTGIETER
Department of Metallurgical and Materials Engineering, Federal University of Technology, Akure, Nigeria2School of Process and Materials Engineering, University of the Witwatersrand, Johannesburg, South Africa
Abstract:
This study investigates the1 effectiveness of gravity concentration for the beneficiation of Itakpe (Nigeria) iron ore achieved through jigging operations. Iron ore obtained from Itapke Iron Ore Mining Project, Kogi State, Nigeria which contains a very high amount of quartz as revealed by x-ray diffraction was crushed using the laboratory dodge crusher and ground in a laboratory ball mill. Particle size analysis was carried out over the range of +4750µm and -75µm in 12 different mesh sizes, and the ore was jigged in a Laboratory Denver Mineral Jig. The operating variables used to determine the recovery effectiveness of jigging include; particle size, dilution ratio and bedding thickness. Recovery of iron ore was assessed by determining the percentages of Fe in the underflow and overflow using the Atomic Absorption spectrometry (AAS) method. Optimum iron ore recovery of 71% was achieved when the jig was operated at medium stroke and speed, with a feed slurry of average dilution and at a particle size of 600µm.
Keywords: Iron ore; Jigging; Particle size; Dilution ratio; Bedding thickness.
INTRODUCTION
The Itakpe iron ore deposit has a reserve of about 200 million tonnes with an average iron ore content of 36%. This has to be beneficiated at a rate of 8 million tonnes per year to produce 64% Fe concentrate as sinter material for the Ajaoukuta blast furnace and 68% Fe concentrate as pellet feed for the direct reduction plant at Aladja, all in Nigeria. At this production rate, large quantities of tailings are obtained as waste product of the beneficiated iron ore (Adepoju and Olaleye 2001).
Since the goal of every mineral processing operation/technique is to effectively separate the valuable material from the gangue with minimum metal loss in tailings, the need to develop and employ a sustainable, effective and relatively economical method of separation is imperative. The concentration of the valuable minerals from the gangue involves exploitation of the differences in the mineral properties of the ore after effective comminution (Akande and Olaleye 2000).
Magnetic separation and flotation are the most widely accepted technologies for the upgrading of iron ore particles, but these processes result in iron concentrate with high amounts of very fine and/or interlocked silica particles (Yang, et al 2003). Mohanty (2002) also found that the ability of flotation to treat mixed-phase (middling) and weakly hydrophobic particles is not satisfactory. To address the aforementioned problems and thus to achieve a higher iron ore recovery, several new attempts and technologies are being developed with an added aim of achieving economical and environmental benefits through use of the jigging method (Parkinson, 1989; Yang, 1996; Honaker, et al, 1996; Galvin et al 2002).
The mineralogical characterization of Itakpe iron ore shows that it contains mainly hematite, magnetite and quartz whose specific gravities give sound basis for adopting the gravity concentration technique. In this study, the effectiveness of gravity techniques for concentrating iron ore from bulk Itakpe (Nigeria) iron ore was studied using the jigging method and the effects of operating variables on the recovery of iron ore using the laboratory Denver Mineral Jig were investigated.
MATERIALS AND METHODS
The bulk ore used in this study was obtained from the Itakpe mine, north of Okene in Kogi State, Middle Belt Region of Nigeria. Its chemical composition as revealed by X-ray fluorescence is as shown in Table1
The bulk ore used in this study was obtained from the Itakpe mine, north of Okene in Kogi State, Middle Belt Region of Nigeria.
The ore was broken into sizes that could be fed into the jaw crusher using a sledgehammer. Crushing was carried out in a laboratory dodge crusher and ground in a laboratory ball mill. Ore sieving was carried out using the laboratory sieve shaker as described by Pryor (1965) and Adepoju & Olaleye (2001) by placing 6000g of the ore in the uppermost ASTM standard sieve. The nest of the ASTM sieves was loaded with the ore and allowed to vibrate for 5 minutes. After the required time, the nest of sieves was taken apart and the amount of material retained on each sieve was weighed. Composition was determined by X-ray fluorescence.
100 grams of the ore of the same size from the product of the sieve analysis was stored in a tray to form the feed or head material for the jigging operation. Steel balls were spread to form a layer on the screen of the mineral jig as a bedding material to varying depths. The spigot of the hutch compartment was plugged with rubber cork and water was added to cover the ragging in the feeding compartment. The head or feed was fed into the feeding compartment.
Feed material mixed with water at varying dilutions was added to the jig and the jigging process was allowed for 4 minutes. At the end of each jigging operation, the spigot of the hutch compartment was opened and the product was collected as the underflow. The overflow materials left in the feeding compartment were scooped and washed out. The two products (underflow and overflow) were dried, weighed and recorded. The experiments were repeated with varying bedding thickness, dilution rates and particle sizes.
The amount of iron ore in each of the underflow and overflows were evaluated by determining the percentages of Fe in the samples using Atomic Absorption Spectrometry (AAS) method. A sample weight of 2g was the standard measurement for this experiment. Samples were dried prior to analysis. Samples were well mixed before weighing to make sure they were homogenous. The samples were digested with 20 ml of .01 M Hydrochloric acid (HCL) solution by shaking in plastic centrifuge tubes for 15-20 minutes. Concentration of each of the samples was measured against standard solutions. The composition of iron and silicon in each sample was then ascertained using a Buck Scientific model 200 atomic absorption spectrophotometer with air-acetylene flame.
RESULTS AND DISCUSSION
The results obtained from the particle size analysis are as shown in Table 2 and the compositional analyses revealed by X-ray are graphically presented in Figures 1, 2 and 3. Tables 3, 4, and 5 show the results obtained from the jigging operations and they are as graphically illustrated in Figures 4, 5 and 6.
Size analysis
It can be observed from Table 2 that the smaller the aperture of the sieve, the lower the weight% of Itakpe iron ore retained. The aperture range of 1180µm has the most retained weight% followed by 850µm and then 2000µm respectively. It was also observed in Table 2 that 2000µm has the most retained quantity of quartz followed by 1180µm then 350µm. The rate of reduction of both iron ore and quartz varies (Figures 1 to 3). Quartz dissipates easily, breaking down to fines with little applied stress. Though the hardness value of quartz (i.e. 7 on the Mohr’s scale) is a bit higher than the iron ore (Hematite (5.5-6.5), Magnetite (5.5-6), it is far more brittle than iron ore (Gribble, 1988).
The disparity in the cumulative weight% retained and cumulative passing of the ore is demonstrated by Figures 1 and 2. From both graphs, it can be seen that the cumulative weight% retained and cumulative passing graphs are inversely proportional to each other. The Gate-Gaudin-Schumann’s representation of sieve analysis in Figure 3 shows that the compositional distributions of the materials are linearly and uniformly distributed over a wide size range.
Separation effectiveness:
The results, as shown in Tables 4, 5, and 6 and Figures 4, 5 and 6, revealed that iron ore concentrate (underflow) was effectively separated from the tailings (overflow), which are essentially quartz. Theoretically, effective separation was possible because the quotient of the difference in their specific gravities is greater than 2.5 (Equation 1)
Effects of bedding thickness
The effects of bedding material and thickness of the bed on the recovery of iron ore are shown in Figures 4, 5 and 6. The bedding material and the thickness of the bedding materials used has an effect on the %Fe recovered as shown in Figure 6. Thick bedding causes more friction action during the suction stage, making concentration less possible. Thin bedding reduces friction during suction and also, allows easy passage of the Fe concentrate during jigging.
Effects of Particle size:
The effect of the particle size on the recovery of iron ore is also shown in Tables 4,5, and 6, and Figures 4, 5 and 6. The highest recovery was achieved at 600µm. Lower separation efficiency in finer particles is believed to be caused by the negligible mass associated with this size particles. Particles so small, that settle in accordance with Stroke’s law, are unsuitable for concentration (Wills, 1989). Lower recovery of Fe at larger particle sizes may be due to the reduced possibilities of the larger particles passing through the jig screen. A coarser particle will have a reduced chance of passing through the jig screen and thereby will report to the overflow as tailings (Mohanty, et al 2002).
CONCLUSION
The results of this project work have clearly shown that effective separation of iron ore concentrate from bulk Itakpe iron ore by jigging operations is possible. It is clearly revealed that the effectiveness of the separation was greatly influenced by the operating variables of the jig and the particle size of the ore. The result of the work has also shown that the optimum Fe recovery could be achieved when the jig is operated at medium stroke and speed under the conditions of a thin bed with feed slurry of average dilution at a particle size range of 600µm.
beneficiation of Low grade iron ore fines
Since a decade the Institute of Minerals and Materials,Technology has been engaged in Iron ore research to enhance raw material quality for Iron and Steel industry. Consequently, from our experience we discuss the characterization and beneficiation aspects of five different types of Iron ore samples—Barbil, Bailladilla, Goa, Barajamda and Hospet (Banded Hematite Quartzite, BHQ).
The characterization studies in general indicate that hematite and goethite are the major Iron bearing minerals, where as kaolinite, gibbsite and quartz are present as the gangue minerals. Particle counts of the close sized fractions indicate that the degree of liberation of hematite is about 87% at 53 micron size. The chemical analysis of the hematite ores on an average conform to 57.8 to 64.5% Fe, 1.56 to 6.5% SiO and 1.3 to 6% Al3O2. These ores subject to beneficiation yield a concentrate containing 61.5 to 66% Fe at 62 to 86% yield. In case of BHQ ore, column flotation technique has been adopted to obtain a concentrate of 66% Fe at 44.7% yield. It has been observed that beneficiation of low grade ores invariably pose specific challenge due to the presence of clayey/earthy materials rich in aluminum. For both hematite as well as BHQ ores proven flow-sheet with material balance has been developed and satisfactorily implemented through our clients. The processes that have been developed are ideal for pellet making where the future of Steel industry rests.
1. Introduction
India occupies sixth position in the world's Iron-ore reserves and is one of the major Iron-ore producer and exporter due to availability of quality ore and skilled mining personnel. India's Iron ore reserve is around 25,249 million tonnes (MT) apart from Banded Hematite-Quartzite (BHQ) and Banded Hematite Jasper (BHJ). Although India is blessed with large reserves of Iron ore containing average grade around 58% Fe, the performance of blast furnaces has been at lower levels in comparison with the developing countries. This has been mainly due to the presence of high levels of impurities such as silica and Alumina in the raw material contradicting to the blast furnace chemistry.
In order to increase the efficiency of blast furnace, some of the issues relating to Iron ores include chemical composition of Iron ore with low Fe content and high Al:Si ratio, low strength, high temperature break down, lower reducibility, low temperature softening and melting behavior of the Iron ores, etc. Normally Iron ores with Fe content above 65% are desirable to achieve better productivity either in blast furnace or direct reduction. The other impurities level such as Na, K, S and P should be as low as possible. Alumina and Silica content should be within permissible limit for better fluidity of slag. Due to non availability of quality Iron ore, the run-off-mne (rom) needed to be beneficiated to lower the impurities to improve the strength of sinter and pellet quality. The physical, chemical and metallurgical properties of lumps, sinters and pellets are important as they have a significant impact on furnace performance.
For economics reasons, quality raw material is not only required for blast furnace operation but also for the emerging technologies such as smelting reduction and direct reduction route. Beside that, India has set itself a target of achieving production capacity of 110 MT of Steel by 2020 and the required quantity of Iron ore is projected at 190 MT. Over the next few years, demand for Indian Iron ore is expected to rise by more than 200 million tonnes per year to meet the internal demand and export. Two major shifts in Iron ore supply for the Indian Iron and Steel industry have occurred. First the export to foreign market owing to liberalization in the economy and second the adaptation of beneficiation and pelletization practices to utilize low-grade ores and fines. In India for economic and industrial growth, a number of Steel plants have been planed in the states of Orissa, Jharkhand, Chhattisgarh, Karnataka and Maharastra. As the quality of raw materials declines, the impact of Iron making processes on pollution control and energy required will worsen in days to come. Most of the rom Iron ore contains lot of impurities that needs beneficiation prior to use. Therefore research on utilization of low grade Iron ore to produce quality raw material would play a key role in future which is a fact acknowledged by the Iron and Steel industry.
Iron ore is being beneficiated all round the world to meet the quality requirement of Iron and Steel industries. However, each source of Iron ore has its own peculiar mineralogical characteristics and requires the specific beneficiation and metallurgical treatment to get the best product out of it. The choice of the beneficiation treatment depends on the nature of the gangue present and its association with the ore structure. Several techniques such as washing, jigging, magnetic separation, advanced gravity separation and flotation are being employed to enhance the quality of the Iron ore. Washing, jigging and classification are being carried out for the beneficiation of Iron ores in India. During washing and sizing of the ore, slimes with less than 0.21 mm size are generated and discarded into the tailing pond. It is estimated that around 10 million tonnes of slimes are being generated in every year during the processing of hematite ore and lost as tailings containing around 48-62% of Fe. However beneficiation and utilization of these slimes still remains as a challenging task.
The ever increasing need to utilize the slimes is being reflected in the shift in Steel production from basic blast furnaces to electric arc furnace technology. In the USA, around 40% of Steel is produced in electric arc furnaces by using Iron ore pellets. Pellets are also used in traditional blast furnaces in some parts of the world. Pellets are ideal material as a feed to direct-reduction Iron plants. However the use of pellet in our Steel plant is very limited. Lot of low grade Iron ore fines are generated during preparation of lumps, calibrated ores and sinter-fines. In addition to these fines, 10-15% of ore mined is generated as slimes and are discarded as tailings. These fines and tailings are potential sources to produce pellet grade concentrate after suitable beneficiation. Another source of pellet feed concentrate is from low and off grade ores such BHQ & BHJ. All these materials can be beneficiated to yield quality pellet feed. Keeping this in observation, detailed studies have been carried out on different source of materials to produce quality concentrates for pellet feed. The results of four different types of hematite ore fines covering from Barbil , Balladilla, Goa, Barjamada have been discussed in this paper. Increasing worldwide demand for Iron ore triggering the development of India's magnetite and BHQ ores of Karnataka for effective utilization is also highlighted.
2. MATERIALS AND METHODS
2.1 Ore samples
Five different types of representative Iron ore samples from Iron ore mines of India through Barbil, Balladilla, Goa, Barjamada and Hospet area were collected and brought to the laboratory for the detail investigation studies. The as received hematite samples which are either fines or slimes were thoroughly mixed and representative sample of each was drawn by conning and quartering method for different characterization, mineralogical and beneficiation studies. The BHQ sample was lumpy variety with little amount of fines. The sample is very hard in nature and compact. The sample colour is grayish black. Similar coning and quartering method was applied to draw a representative sample from the bulk sample for different investigations.
2.2 Experimental
2.2.1 Physical and Chemical Characterization Studies:
The size analysis of the received Iron ore sample, were carried out by wet sieving techniques to know the average particle size of the sample. The different size fractions thus obtained were subjected to chemical analysis to ascertain the different quantitative elemental composition of the sample. The complete chemical analysis of the rom ore and different size fractions were carried out by X-ray florescence technique and wet chemical analysis. The XRF analyses were carried out against the standard calibrated samples of similar values. The loss on ignition (LOI) of Iron ore samples was determined by igniting around 2.0 gm of sample at about 1000 C for four hours in a muffle furnace in silica crucible.
Closed sized classified samples were examined under stereomicroscope by preparing the corresponding grain slides for identification of different minerals. The X-ray diffraction studies of selected samples were also carried out using a Philips model diffractmeter with CuK radiation. The bulk sample was crushed to below 1 mm size and wet sieved into different size fractions. The size fractions were mounted in resin with hardner and polished following standard procedures. The polished sections were studied under reflected light microscope and the particles of different typologies were counted.
2.2.3 Grinding Studies
In order to increase the grade of Iron ore and for the subsequent liberation of Iron values from the locked particles, the samples were subjected to wet grinding to generate different size particles. A standard ball mill of 12”x12” with required weight of balls as per Bonds formula at 45% filling was used. The grinding was carried out in batch prior to different beneficiation studies. The objective is to achieve the maximum liberation of the Iron particles from the associated gangues due to reduction in size. The large-scale continuous grinding studies were also carried out using 24?x 24? ball mill to produce samples for further investigations and to establish grinding parameters. All the grinding studies were carried out at 40% solids consistency in the ball mill.
2.2.4 Beneficiation Studies
Beneficiation studies using various techniques such as hydrocyclone, spiral, magnetic separation, flotation etc. were carried out to develop a suitable process flowsheet as a step towards the up-gradation of Iron values and to reduce the gangue content. The required separation technique was selected based on particle size and the properties for effective separation. Initially, the hematite ore fines were ground to required size and then subjected to separation. The sample was ground to below 1.0 mm size for hematite ore fines and less than 100 microns for BHQ ore. However, the tailing samples from Goa ore have been treated without further grinding.
2.2.4.1 Hydrocyclone
Hydrocyclone was used as a step towards the up-gradation of Iron values as well as for de-sliming of slime particles present in the sample. Since the ground samples contained large amount of slime materials comprising of particles down to sub micron size, de-sliming was also thought of prior to flotation. The effect of some of the variables namely apex diameter, vortex finder diameter, operating pressure, solids concentration etc. were optimized. The throughput of hydro-cyclone during the experiments was 240 to 780 kg/hr solids and the solid concentrations of ~10-20 % by weight were maintained during the course of the experiments. The underflow and overflow materials were collected at a steady state for a fixed time, dried, weighed and analyzed for the desired Iron and other constituents. The overflow & underflow samples collected at optimum operating conditions were used for further studies
2.2.4.2 Spiral
The spiral concentrator of 100 mm dia. was used to enrich the Iron content of the classified sample (hydrocyclone underflow). The spiral is an energy saving gravity equipment where large quantities of sample can be fed for pre-concentrations. In the spiral study, the Iron ore sample was fed to the centrifugal pump at the required solids consistency and the slurry was kept re-circulating for a predetermined time. The entire concentrate and tailings were collected after attaining the steady state. The concentrates in some cases were cleaned to improve the grade of products. All the products thus obtained were dried, weighed, and analyzed.
2.2.4.3 Wet High Intensity Magnetic Separation
The wet high intensity magnetic separator (WHIMS) and high gradient magnetic separator (HGMS) were used at different magnetic field intensities to recover the fine Iron values from the hydrocyclone over flow or spiral tailings. Both the separators have provision for different magnetic groves of width and matrix with variable currents to provide different magnetic intensities. A desired concentration of solids was passed through the magnetic separator. In some cases the magnetic products were cleaned in second stage to enhance the quality of the product from first stage separation.
2.2.4.4 Flotation
Batch flotation studies were carried out to select either direct or reverse flotation technique to optimize reagent combination and to establish the number of stages of operations. Denver D-12 sub-aeration flotation machine was used for the batch flotation studies. Both cationic (dodecylamine) and anionic (oleic acid) reagents were used as collectors while MIBC was used as the frother. The flotation conditions were optimized to get good grade concentrate with high recoveries. The column flotation studies were carried out by using 100 mm diameter glass column designed and fabricated at our laboratory. The column was operated at nominal capacity of 20 kg of Iron ore fines per hour with the help of a peristaltic pump. Both the concentrate and tailings were collected separately after attaining the steady state and analyzed for Iron content.
From steel world
The characterization studies in general indicate that hematite and goethite are the major Iron bearing minerals, where as kaolinite, gibbsite and quartz are present as the gangue minerals. Particle counts of the close sized fractions indicate that the degree of liberation of hematite is about 87% at 53 micron size. The chemical analysis of the hematite ores on an average conform to 57.8 to 64.5% Fe, 1.56 to 6.5% SiO and 1.3 to 6% Al3O2. These ores subject to beneficiation yield a concentrate containing 61.5 to 66% Fe at 62 to 86% yield. In case of BHQ ore, column flotation technique has been adopted to obtain a concentrate of 66% Fe at 44.7% yield. It has been observed that beneficiation of low grade ores invariably pose specific challenge due to the presence of clayey/earthy materials rich in aluminum. For both hematite as well as BHQ ores proven flow-sheet with material balance has been developed and satisfactorily implemented through our clients. The processes that have been developed are ideal for pellet making where the future of Steel industry rests.
1. Introduction
India occupies sixth position in the world's Iron-ore reserves and is one of the major Iron-ore producer and exporter due to availability of quality ore and skilled mining personnel. India's Iron ore reserve is around 25,249 million tonnes (MT) apart from Banded Hematite-Quartzite (BHQ) and Banded Hematite Jasper (BHJ). Although India is blessed with large reserves of Iron ore containing average grade around 58% Fe, the performance of blast furnaces has been at lower levels in comparison with the developing countries. This has been mainly due to the presence of high levels of impurities such as silica and Alumina in the raw material contradicting to the blast furnace chemistry.
In order to increase the efficiency of blast furnace, some of the issues relating to Iron ores include chemical composition of Iron ore with low Fe content and high Al:Si ratio, low strength, high temperature break down, lower reducibility, low temperature softening and melting behavior of the Iron ores, etc. Normally Iron ores with Fe content above 65% are desirable to achieve better productivity either in blast furnace or direct reduction. The other impurities level such as Na, K, S and P should be as low as possible. Alumina and Silica content should be within permissible limit for better fluidity of slag. Due to non availability of quality Iron ore, the run-off-mne (rom) needed to be beneficiated to lower the impurities to improve the strength of sinter and pellet quality. The physical, chemical and metallurgical properties of lumps, sinters and pellets are important as they have a significant impact on furnace performance.
For economics reasons, quality raw material is not only required for blast furnace operation but also for the emerging technologies such as smelting reduction and direct reduction route. Beside that, India has set itself a target of achieving production capacity of 110 MT of Steel by 2020 and the required quantity of Iron ore is projected at 190 MT. Over the next few years, demand for Indian Iron ore is expected to rise by more than 200 million tonnes per year to meet the internal demand and export. Two major shifts in Iron ore supply for the Indian Iron and Steel industry have occurred. First the export to foreign market owing to liberalization in the economy and second the adaptation of beneficiation and pelletization practices to utilize low-grade ores and fines. In India for economic and industrial growth, a number of Steel plants have been planed in the states of Orissa, Jharkhand, Chhattisgarh, Karnataka and Maharastra. As the quality of raw materials declines, the impact of Iron making processes on pollution control and energy required will worsen in days to come. Most of the rom Iron ore contains lot of impurities that needs beneficiation prior to use. Therefore research on utilization of low grade Iron ore to produce quality raw material would play a key role in future which is a fact acknowledged by the Iron and Steel industry.
Iron ore is being beneficiated all round the world to meet the quality requirement of Iron and Steel industries. However, each source of Iron ore has its own peculiar mineralogical characteristics and requires the specific beneficiation and metallurgical treatment to get the best product out of it. The choice of the beneficiation treatment depends on the nature of the gangue present and its association with the ore structure. Several techniques such as washing, jigging, magnetic separation, advanced gravity separation and flotation are being employed to enhance the quality of the Iron ore. Washing, jigging and classification are being carried out for the beneficiation of Iron ores in India. During washing and sizing of the ore, slimes with less than 0.21 mm size are generated and discarded into the tailing pond. It is estimated that around 10 million tonnes of slimes are being generated in every year during the processing of hematite ore and lost as tailings containing around 48-62% of Fe. However beneficiation and utilization of these slimes still remains as a challenging task.
The ever increasing need to utilize the slimes is being reflected in the shift in Steel production from basic blast furnaces to electric arc furnace technology. In the USA, around 40% of Steel is produced in electric arc furnaces by using Iron ore pellets. Pellets are also used in traditional blast furnaces in some parts of the world. Pellets are ideal material as a feed to direct-reduction Iron plants. However the use of pellet in our Steel plant is very limited. Lot of low grade Iron ore fines are generated during preparation of lumps, calibrated ores and sinter-fines. In addition to these fines, 10-15% of ore mined is generated as slimes and are discarded as tailings. These fines and tailings are potential sources to produce pellet grade concentrate after suitable beneficiation. Another source of pellet feed concentrate is from low and off grade ores such BHQ & BHJ. All these materials can be beneficiated to yield quality pellet feed. Keeping this in observation, detailed studies have been carried out on different source of materials to produce quality concentrates for pellet feed. The results of four different types of hematite ore fines covering from Barbil , Balladilla, Goa, Barjamada have been discussed in this paper. Increasing worldwide demand for Iron ore triggering the development of India's magnetite and BHQ ores of Karnataka for effective utilization is also highlighted.
2. MATERIALS AND METHODS
2.1 Ore samples
Five different types of representative Iron ore samples from Iron ore mines of India through Barbil, Balladilla, Goa, Barjamada and Hospet area were collected and brought to the laboratory for the detail investigation studies. The as received hematite samples which are either fines or slimes were thoroughly mixed and representative sample of each was drawn by conning and quartering method for different characterization, mineralogical and beneficiation studies. The BHQ sample was lumpy variety with little amount of fines. The sample is very hard in nature and compact. The sample colour is grayish black. Similar coning and quartering method was applied to draw a representative sample from the bulk sample for different investigations.
2.2 Experimental
2.2.1 Physical and Chemical Characterization Studies:
The size analysis of the received Iron ore sample, were carried out by wet sieving techniques to know the average particle size of the sample. The different size fractions thus obtained were subjected to chemical analysis to ascertain the different quantitative elemental composition of the sample. The complete chemical analysis of the rom ore and different size fractions were carried out by X-ray florescence technique and wet chemical analysis. The XRF analyses were carried out against the standard calibrated samples of similar values. The loss on ignition (LOI) of Iron ore samples was determined by igniting around 2.0 gm of sample at about 1000 C for four hours in a muffle furnace in silica crucible.
Closed sized classified samples were examined under stereomicroscope by preparing the corresponding grain slides for identification of different minerals. The X-ray diffraction studies of selected samples were also carried out using a Philips model diffractmeter with CuK radiation. The bulk sample was crushed to below 1 mm size and wet sieved into different size fractions. The size fractions were mounted in resin with hardner and polished following standard procedures. The polished sections were studied under reflected light microscope and the particles of different typologies were counted.
2.2.3 Grinding Studies
In order to increase the grade of Iron ore and for the subsequent liberation of Iron values from the locked particles, the samples were subjected to wet grinding to generate different size particles. A standard ball mill of 12”x12” with required weight of balls as per Bonds formula at 45% filling was used. The grinding was carried out in batch prior to different beneficiation studies. The objective is to achieve the maximum liberation of the Iron particles from the associated gangues due to reduction in size. The large-scale continuous grinding studies were also carried out using 24?x 24? ball mill to produce samples for further investigations and to establish grinding parameters. All the grinding studies were carried out at 40% solids consistency in the ball mill.
2.2.4 Beneficiation Studies
Beneficiation studies using various techniques such as hydrocyclone, spiral, magnetic separation, flotation etc. were carried out to develop a suitable process flowsheet as a step towards the up-gradation of Iron values and to reduce the gangue content. The required separation technique was selected based on particle size and the properties for effective separation. Initially, the hematite ore fines were ground to required size and then subjected to separation. The sample was ground to below 1.0 mm size for hematite ore fines and less than 100 microns for BHQ ore. However, the tailing samples from Goa ore have been treated without further grinding.
2.2.4.1 Hydrocyclone
Hydrocyclone was used as a step towards the up-gradation of Iron values as well as for de-sliming of slime particles present in the sample. Since the ground samples contained large amount of slime materials comprising of particles down to sub micron size, de-sliming was also thought of prior to flotation. The effect of some of the variables namely apex diameter, vortex finder diameter, operating pressure, solids concentration etc. were optimized. The throughput of hydro-cyclone during the experiments was 240 to 780 kg/hr solids and the solid concentrations of ~10-20 % by weight were maintained during the course of the experiments. The underflow and overflow materials were collected at a steady state for a fixed time, dried, weighed and analyzed for the desired Iron and other constituents. The overflow & underflow samples collected at optimum operating conditions were used for further studies
2.2.4.2 Spiral
The spiral concentrator of 100 mm dia. was used to enrich the Iron content of the classified sample (hydrocyclone underflow). The spiral is an energy saving gravity equipment where large quantities of sample can be fed for pre-concentrations. In the spiral study, the Iron ore sample was fed to the centrifugal pump at the required solids consistency and the slurry was kept re-circulating for a predetermined time. The entire concentrate and tailings were collected after attaining the steady state. The concentrates in some cases were cleaned to improve the grade of products. All the products thus obtained were dried, weighed, and analyzed.
2.2.4.3 Wet High Intensity Magnetic Separation
The wet high intensity magnetic separator (WHIMS) and high gradient magnetic separator (HGMS) were used at different magnetic field intensities to recover the fine Iron values from the hydrocyclone over flow or spiral tailings. Both the separators have provision for different magnetic groves of width and matrix with variable currents to provide different magnetic intensities. A desired concentration of solids was passed through the magnetic separator. In some cases the magnetic products were cleaned in second stage to enhance the quality of the product from first stage separation.
2.2.4.4 Flotation
Batch flotation studies were carried out to select either direct or reverse flotation technique to optimize reagent combination and to establish the number of stages of operations. Denver D-12 sub-aeration flotation machine was used for the batch flotation studies. Both cationic (dodecylamine) and anionic (oleic acid) reagents were used as collectors while MIBC was used as the frother. The flotation conditions were optimized to get good grade concentrate with high recoveries. The column flotation studies were carried out by using 100 mm diameter glass column designed and fabricated at our laboratory. The column was operated at nominal capacity of 20 kg of Iron ore fines per hour with the help of a peristaltic pump. Both the concentrate and tailings were collected separately after attaining the steady state and analyzed for Iron content.
From steel world
2009/04/15
British Mining Companies Face Challenge On Unjust Practices
Representatives of communities in Colombia, West Papua and the USA are in London to challenge the claims of two of Britain’s biggest mining companies that their operations are sustainable and fair. Critics say that in reality they are violating human rights and causing irreversible environmental damage.
They lobbyists will speak at a press briefing at Amnesty UK’s Human Rights Action Centre the evening before both companies hold their shareholders’ meetings.
Mining stocks have plummeted in recent months and both Rio Tinto and Anglo American have suffered major writedowns in their market capitalisation. The Church of England is a major shareholder in both companies and had a combined investment in the two companies of £120 million according to the last annual report and accounts from the Church Commissioners.
Less well-known in Britain are the impacts of the companies’ activities on the people around their operations, say campaigners.
Anglo American continues to be criticised for the way in which it has removed farming communities from their lands in Colombia and South Africa; for its failure to gain the indigenous peoples’ permission to operate on their land in the Philippines; for the potential impacts of its proposed Pebble Mine in Alaska on water quality and salmon fisheries; and on the potential impact of its water use in Peru.
Rio Tinto is under fire for its part in the massive damage done by the Grasberg copper and gold mine in West Papua and the multiple violations of human and indigenous rights carried out by the Indonesian military to protect the mine; for its involvement in a joint venture with Muriel Mining in Colombia which is exploring for copper and gold on indigenous land against the express wishes of indigenous people, against a background of militarization and human rights abuses; for pollution round its subsidiary Kennecott’s copper mine in Utah and the potentially catastrophic impact of its proposed Eagle Mine on water quality in Michigan’s Upper Peninsula; and for the legacy of its Capper Pass tin smelter in Hull.
The London Mining Network (LMN), a coalition of environmental, development and human rights groups, is calling on the UK government to ensure that all UK businesses are accountable for their impact on communities around the world. LMN is concerned about the power exerted by UK mining companies over their host countries’ governments and local communities.
London is the mining capital of the world: it is home to many of the largest mining companies’ headquarters and the backdrop to the majority of both their investment and metals trading activity.
“The Rev Jon Magnuson and I feel compelled to travel to London to speak before the Rio Tinto board because the company continues to push its Eagle Mine project through despite wide-spread opposition from the people of the Great Lakes region. Our community remains steadfast in challenging the company’s fraudulent and ill-designed mine application and the company’s complete disregard for human health, freshwater, and Native American treaty rights,” said Fr Henry Ramirez Soler from the Colombian Inter-church Committee for Justice and Peace.
The Committee works with indigenous communities affected by exploration for copper and gold in the province of Choco, Colombia, in which Rio Tinto is involved.
Richard Solly, Secretary of the London Mining Network commented: “Mining companies have to clean up their act. They are better at talk than action. The UK Government has to hold to account companies based here or raising money on the London Stock Exchange. Everybody now knows the disastrous consequences of under-regulation of financial services. Well, much of the damaging investment that banks, insurance companies, pension funds and hedge funds have made has been in destructive mining projects.
Source: Ekklesia
http://paguntaka.org/2009/04/12/british-mining-companies-face-challenge-on-unjust-practices/
They lobbyists will speak at a press briefing at Amnesty UK’s Human Rights Action Centre the evening before both companies hold their shareholders’ meetings.
Mining stocks have plummeted in recent months and both Rio Tinto and Anglo American have suffered major writedowns in their market capitalisation. The Church of England is a major shareholder in both companies and had a combined investment in the two companies of £120 million according to the last annual report and accounts from the Church Commissioners.
Less well-known in Britain are the impacts of the companies’ activities on the people around their operations, say campaigners.
Anglo American continues to be criticised for the way in which it has removed farming communities from their lands in Colombia and South Africa; for its failure to gain the indigenous peoples’ permission to operate on their land in the Philippines; for the potential impacts of its proposed Pebble Mine in Alaska on water quality and salmon fisheries; and on the potential impact of its water use in Peru.
Rio Tinto is under fire for its part in the massive damage done by the Grasberg copper and gold mine in West Papua and the multiple violations of human and indigenous rights carried out by the Indonesian military to protect the mine; for its involvement in a joint venture with Muriel Mining in Colombia which is exploring for copper and gold on indigenous land against the express wishes of indigenous people, against a background of militarization and human rights abuses; for pollution round its subsidiary Kennecott’s copper mine in Utah and the potentially catastrophic impact of its proposed Eagle Mine on water quality in Michigan’s Upper Peninsula; and for the legacy of its Capper Pass tin smelter in Hull.
The London Mining Network (LMN), a coalition of environmental, development and human rights groups, is calling on the UK government to ensure that all UK businesses are accountable for their impact on communities around the world. LMN is concerned about the power exerted by UK mining companies over their host countries’ governments and local communities.
London is the mining capital of the world: it is home to many of the largest mining companies’ headquarters and the backdrop to the majority of both their investment and metals trading activity.
“The Rev Jon Magnuson and I feel compelled to travel to London to speak before the Rio Tinto board because the company continues to push its Eagle Mine project through despite wide-spread opposition from the people of the Great Lakes region. Our community remains steadfast in challenging the company’s fraudulent and ill-designed mine application and the company’s complete disregard for human health, freshwater, and Native American treaty rights,” said Fr Henry Ramirez Soler from the Colombian Inter-church Committee for Justice and Peace.
The Committee works with indigenous communities affected by exploration for copper and gold in the province of Choco, Colombia, in which Rio Tinto is involved.
Richard Solly, Secretary of the London Mining Network commented: “Mining companies have to clean up their act. They are better at talk than action. The UK Government has to hold to account companies based here or raising money on the London Stock Exchange. Everybody now knows the disastrous consequences of under-regulation of financial services. Well, much of the damaging investment that banks, insurance companies, pension funds and hedge funds have made has been in destructive mining projects.
Source: Ekklesia
http://paguntaka.org/2009/04/12/british-mining-companies-face-challenge-on-unjust-practices/
2009/04/11
Subscribe to:
Posts (Atom)